Abstract
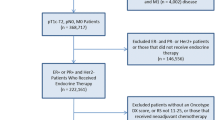
The Oncotype DX recurrence score (RS) reduces breast cancer adjuvant treatment utilization, but the reasons for this effect are not straightforward. We performed a retrospective chart review of 89 consecutive node-negative breast cancer patients for whom RS was ordered to facilitate adjuvant treatment decisions. By subtracting the relapse rate predicted by RS from that calculated using the Adjuvant! Online (AOL) web-based instrument, a “prognostic delta” (P∆) was determined, reflecting the difference between prognoses predicted by these two indices. Clinician interviews were conducted to evaluate the actual effect of RS on treatment decisions and its relation to P∆. Adjuvant chemotherapy use decreased from 61 to 26 % as a consequence of RS results (p < 0.0001). In multivariate analysis, RS was the only factor significantly associated with the final adjuvant treatment choice. Surprisingly, RS caused chemotherapy to be withheld even when P∆ was negative (i.e., cases in which RS predicted a less favorable outcome than AOL). The prognostic and chemotherapy predictive utilities of the RS do not fully account for its effect in reducing adjuvant chemotherapy use. Further studies are required to more fully elucidate other factors that may be responsible for this effect, including the possibility of unintended influence.





Similar content being viewed by others
References
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826
Sparano JA, Paik S (2008) Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol 26:721–728
Paik S, Tang G, Shak S et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734
Habel LA, Shak S, Jacobs MK et al (2006) A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 8:R25
Goldstein LJ, Gray R, Badve S et al (2008) Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol 26:4063–4071
Esteva FJ, Sahin AA, Cristofanilli M et al (2005) Prognostic role of a multigene reverse transcriptase-PCR assay in patients with node-negative breast cancer not receiving adjuvant systemic therapy. Clin Cancer Res 11:3315–3319
Febbo PG, Ladanyi M, Aldape KD et al (2011) NCCN task force report: evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw 9(Suppl 5):S1–S32 Quiz S33
Harris L, Fritsche H, Mennel R et al (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25:5287–5312
Genomic Health Corporation (2011) Genomic health investor presentation (monograph online). http://investor.genomichealth.com/events.cfm. Accessed 24 Aug 2011
Hornberger J, Chien R (2010) Meta-analysis of the decision impact of the 21-gene breast cancer recurrence score in clinical practice [abstract]. Cancer Res 70(S24):S210
Lyman GH, Cosler LE, Kuderer NM et al (2007) Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer 109:1011–1018
Klang SH, Hammerman A, Liebermann N et al (2010) Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health 13:381–387
Lamond NWD, Skedgel C, Rayson D, Lethbridge L, Younis T (2012) Cost utility of the 21 gene recurrence score assay in node-negative and node-positive breast cancer. Breast Cancer Res Treat. doi:10.1007/s10549-012-1989-5
Sparano JA (2006) TAILORx: trial assigning individualized options for treatment (Rx). Clin Breast Cancer 7:347–350
Ravdin PM, Siminoff LA, Davis GJ et al (2001) Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 19:980–991
Fisher B, Dignam J, Wolmark N et al (1997) Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst 89:1673–1682
Early Breast Cancer Clinical Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Henry LR, Stojadinovic A, Swain SM et al (2009) The influence of a gene expression profile on breast cancer decisions. J Surg Oncol 99:319–323
Lo SS, Mumby PB, Norton J et al (2010) Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol 28:1671–1676
Asad J, Jacobson AF, Estabrook A et al (2008) Does oncotype DX recurrence score affect the management of patients with early-stage breast cancer? Am J Surg 196:527–529
Dowsett M, Cuzick J, Wale C et al (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28:1829–1834
Dowsett M, Pineda S, Cuzick J (2010) Reply to M. Rossman et al. J Clin Oncol 28(31):e648
Oratz R, Paul D, Cohn AL et al (2007) Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. J Oncol Pract 3:182–186
Ademuyiwa FO, Miller A, O’Connor T, Edge SB, Thorat MA, Sledge GW, Levine E, Badve S (2011) The effects of oncotype DX recurrence scores on chemotherapy utilization in a multi-institutional breast cancer cohort. Breast Cancer Res Treat 126:797–802. doi:10.1007/s10549-010-1329-6
Levine MN, Pritchard KI, Bramwell VH et al (2005) Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol 23:5166–5170
Munzone E, Curigliano G, Burstein HJ et al (2012) CMF revisited in the 21st century. Ann Oncol 23:305–311
Albain KS, Barlow WE, Ravdin PM et al (2009) Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine responsive, node-positive breast cancer: a phase 3 open label, randomised controlled trial. Lancet 374:2055–2063
Tang G, Cuzick J, Costantino JP et al (2011) Risk of recurrence and chemotherapy benefit for patients with node-negative, estrogen receptor-positive breast cancer: recurrence score alone and integrated with pathologic and clinical factors. J Clin Oncol 29:4365–4372
Nahta R, O’Regan RM (2012) Therapeutic implications of receptor signaling in HER2-psotive breast cancers. Breast Can Res Treat. doi:10.1007/s10549-012-2067-8
Osborne CK, Schiff R (2011) Mechanisms of endocrine resistance in breast cancer. Ann Rev Med 62:233–247
Ito T, Kamijo S, Izumi H, Kohno K, Amano J, Ito K (2012) Alteration of Y-box binding protein-1 expression modifies the response to endocrine therapy in estrogen receptor-positive breast cancer. Breast Can Res Treat 133:145–159
Acknowledgments
We thank biostatistician Martin Feuerman, MS for providing his insightful review of our data. Special thanks also to the medical oncologists who generously gave their time as interviewees so that we could best understand the factors essential to the decision-making process for each of their patients in this study—Nina D’Abreo MD, Michael Garrison MD, Alexander Hindenburg MD FACP, and Harry Staszewski, MD FACP. Finally, we thank Julie Mischo RN MBA for her organizational assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schneider, J.G., Khalil, D.N. Why does Oncotype DX recurrence score reduce adjuvant chemotherapy use?. Breast Cancer Res Treat 134, 1125–1132 (2012). https://doi.org/10.1007/s10549-012-2134-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2134-1