Abstract
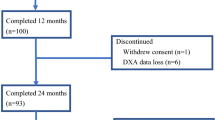
The adjuvant use of aromatase inhibitors in breast cancer is associated with adverse effects on bone health. We previously reported a decline in bone mineral density (BMD) following the switch from tamoxifen to exemestane in the Intergroup Exemestane Study (IES). Here we report effects of endocrine treatment withdrawal on BMD, bone turnover markers (BTM) and fracture rates. 4,724 patients took part in IES, and 206 patients were included in a bone sub-study. BMD and BTM were assessed pre-randomization, during and after the end of treatment (EOT). To evaluate treatment withdrawal effects, 12- and 24-month post EOT BMD results are available for 122 and 126 patients, respectively. Similar patient numbers had BTM measured post EOT. Following treatment withdrawal, the differences in BMD observed between the two endocrine strategies were partially reversed. At 24 months from EOT, spine BMD increased by 1.53% (95%CI 0.63–2.43; p = 0.001) after stopping exemestane and fell by 1.93% (95%CI −2.91 to 0.95; p = 0.0002) following tamoxifen withdrawal. A similar pattern of changes was observed at the hip. At 2 years post EOT, BMD changes from baseline were similar with both treatment strategies. Corresponding inverse changes in BTM were seen, with an increase following tamoxifen withdrawal and a reduction after exemestane. A higher number of fractures occured during exemestane treatment, but fracture rates were similar after treatment withdrawal. With the switch strategy used in IES, the on treatment adverse bone effects of exemestane are reversed. Ongoing monitoring of BMD is therefore not routinely required.






Similar content being viewed by others
References
ATAC Trialists’ Group (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60–62
The Breast International (BIG) I-98 Collaborative Group (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747–2757
Coombes RC, Hall E, Gibson L, Paridaens R, Jassem J, Delozier T et al (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092
Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C et al (2005) Switching of postmenopausal women with endocrine responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366:455–462
Din OS, Dodwell D, Wakefield RJ, Coleman RE (2010) Aromatase inhibitor-induced arthralgia in early breast cancer: what do we know and how can we find out more? Breast Cancer Res Treat 120:525–538
Coleman RE, Body JJ, Gralow JR, Lipton A (2008) Bone loss in patients with breast cancer receiving aromatase inhibitors and associated treatment strategies. Cancer Treat Rev 34(Suppl 1):S31–S42
Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, on behalf of the Intergroup Exemestane Study et al (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369:559–570
Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group, Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9:45–53
Coleman RE, Banks LM, Girgis SI, Kilburn LS et al (2007) Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol 8:119–127
Eastell R, Adams JE, Coleman RE, Howell A et al (2008) Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 26:1051–1057
Coleman RE, on behalf of the ATAC Trialists’ Group (2008) Long-term effects of anastrozole on bone mineral density: seven-year results from the ATAC trial. J Clin Oncol 26:15S Abstract 587
Perez EA, Josse RG, Pritchard KI, Ingle JI, Martino S, Findlay B et al (2006) Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol 24:3629–3635
Reid DM, Doughty J, Eastell R, Heys SD et al (2008) Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev 34(Suppl 1):S3–S18
Hadji P, Body J-J, Aapro MS, Brufsky A et al (2008) Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann Oncol 19:1407–1416
Hines Sl, Jorn HK, Thompson KM, Larson JM (2010) Breast cancer survivors and vitamin D: a review. Nutrition 26:255–262
Acknowledgments
We would like to thank the following investigators who also contributed patients to this study. J Chirgwin, Box Hill Hospital and Maroondah Hospital, Victoria, Australia; L Petruzelka, University Hospital, Prague, Czech Republic; P Redmond, Cork University Hospital, Cork, Ireland; A Hill, St. Vincent’s Hospital, Dublin, Ireland; V Harvey, Auckland Hospital, Auckland, New Zealand; M Krzakowski, Centrum Onkologii, Warszawa, Poland; P Tomczak, Klinika Onkologii Akademii Medycznej W Poznaniu, Poznan, Poland; P Simmonds, Southampton General Hospital, Southampton, UK; S Goodman, Yeovil District Hospital, Somerset, UK; AU Buzdar, University of Texas M. D. Anderson Cancer Center, Houston, USA. We would also like to thank Jazz Heer, Suzanne Hodgson, Joy Liao, Deirdre Price (Central Evaluation Facility), George Baffoe, Saro Niththyananthan (Bone Markers Unit) and Iva Amoako, Claire Snowdon, Sandy Beare, Lorna Gibson (Coordinating Data Centre) of Imperial College London, UK; Pfizer for their continued support and funding; the Independent Data Monitoring Committee (IDMC); and especially, the women who participated in this study.
Conflict of interest
R. E. Coleman: Honoraria—Pfizer.
L. M. Banks: Research Funding—Pfizer.
S. I. Girgis: None.
E. Vrdoljak: Honoraria—Pfizer.
J. Fox: None.
S. J. Cawthorn: None.
R. C. Coombes: Honoraria—Pfizer, Research Funding—Pfizer.
A. Patel: Honoraria—Pfizer, Research Funding—Pfizer.
J. M. Bliss: Honoraria—Pfizer, Research Funding—Pfizer.
L. S. Kilburn: None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted on behalf of the Intergroup Exemestane Study (IES) group.
Rights and permissions
About this article
Cite this article
Coleman, R.E., Banks, L.M., Girgis, S.I. et al. Reversal of skeletal effects of endocrine treatments in the Intergroup Exemestane Study. Breast Cancer Res Treat 124, 153–161 (2010). https://doi.org/10.1007/s10549-010-1121-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1121-7