Abstract
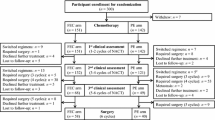
The purpose of this study is to compare the efficacy of weekly paclitaxel to every-3-week schedule in terms of pathologic response and toxicity which caused treatment delay in primary chemotherapy of breast cancer. After pretreatment of two cycles of cyclophosphamide/ pirarubicin/ fluorouracil (cyclophosphamide 500 mg/m2 days 1, 8; pirarubicin 35 mg/m2 days 1, 8; 5-Fu 200 mg/m2 day ci day 1–28, every 4 weeks), 219 women with histologically confirmed T1–3 N0–2 M0 invasive breast cancer, whose vertical diameters production of breast tumor reduced not more than 75%, were randomized to receive four cycles of Pq3wC (arm A: paclitaxel 175 mg/m2 day 1, carboplatin AUC 6 d1, every 3 weeks) or Pq1wC (arm B: paclitaxel 60 mg/m2 days 1, 8, 15, carboplatin AUC 6 day 1 for every 3 weeks) before surgery, stratified by partial or no response (stable disease and progression of disease) evaluated by ultrasonography. Pathologic response of the primary tumor was assessed by using Miller and Payne grading system. We defined grade 4/5 as excellent response, grade 3/4/5 as response and treatment delay as paclitaxel administration being delayed at least 1 week because of toxicity in this study. 213 patients (2 cases with concurrent bilateral breast cancer) were eligible for analysis, 109 patients with 110 lesions in arm A and 104 patients with 105 lesions in arm B. Patients in arm B had a higher excellent pathologic response rate and a higher pathologic response rate compared with patients in arm A (59.0 vs. 45.5%, P = 0.046 and 86.7 vs. 71.8%, P = 0.007). Pathologic complete response (pCR) rate in breast alone was similar between two arms (P = 0.733), but there was a higher pCR rate in patients with partial response to two cycles of cyclophosphamide/pirarubicin/fluorouracil than those with no response (32.4 vs. 13.9%, P = 0.001). There was no treatment-related death, however more patients in arm B than in arm A experienced treatment delay caused by toxicity (60.6 vs. 11.9%, P < 0.001). Under the condition of same cumulative doses, weekly paclitaxel was more effective than 3 weeks schedule in terms of pathologic response to primary chemotherapy in breast cancer, and caused more treatment delay related to toxicity though well tolerant.

Similar content being viewed by others
References
Bear HD (1998) Indications for neoadjuvant chemotherapy for breast cancer. Semin Oncol 25((2 Suppl 3)):3–12
Hortobagyi GN (1990) Comprehensive management of locally advanced breast cancer. Cancer 66(Suppl 6):1387–1391
Rastogi Priya, Anderson StewartJ, Bear HarryD et al (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26:778–785
Van der Hage JA, van de Velde CJ, Julien JP et al (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 19:4224–4237
Henderson IC, Berry DA, Demetri GD et al (2003) Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21(6):976–983
Nabholtz J, Pienkowski T, Mackey J et al (2002) Phase III trial comparing TAC(docetaxel, doxorubicin, cyclophosphamide) with FAC (5-fluorouracil, doxorubicin, cyclophosphamide) in the adjuvant treatment of node positive breast cancer (BC) patients: interim analysis of the BCIRG 001 study (abstract). Proc Am Soc Clin Oncol 21(1):36a
Smith IC, Heys SD, Hutcheon AW et al (2002) Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20:1456–1466
Seidman AD, Berry D, Cirrincione C et al (2004) CALGB 9840: phase III study of weekly (W) paclitaxel (P) via 1-hour infusion versus standard (S) 3 h infusion every third week in the treatment of metastatic breast cancer (MBC), with trastuzumab (T) for HER2 positive MBC and randomized for T in HER2 normal MBC. Proc Am Soc Clin Oncol 22(Suppl 6s) (abstract)
Goldhirsch A, Glick JH, Gelber RD et al (2005) Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 16:1569–1583
Yang WT, Dryden MJ, Gwyn K et al (2006) Imaging of breast cancer diagnosed and treated with chemotherapy during pregnancy. Radiology 239:52–60
Ogston KN, Miller ID, Payne S et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. The Breast 12:320–327
Liedtke C, Mazouni C, Hess KR et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26(8):1275–1281
Green MC, Buzdar AU, Smith T et al (2005) Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 23:5983–5992
Seidman AD, Berry D, Cirrincione C et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors, random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer, Leukemia Group B protocol 9840. J Clin Oncol 26(10):1642–1649
Sparano JA, Wang M, Martino S et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qi, M., Li, J.F., Xie, Y.T. et al. Weekly paclitaxel improved pathologic response of primary chemotherapy compared with standard 3 weeks schedule in primary breast cancer. Breast Cancer Res Treat 123, 197–202 (2010). https://doi.org/10.1007/s10549-010-1000-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1000-2