Abstract
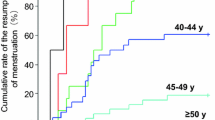
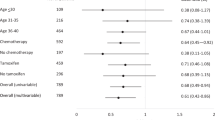
The NSABP B-30 trial addresses whether amenorrhea after adjuvant chemotherapy increases survival. Preliminary to the trial outcome analysis, we examined the incidence of amenorrhea and its relationship to symptoms and quality of life (QOL) in the standard-care arm of this adjuvant breast cancer trial. Premenopausal women treated on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm were included. Questionnaires assessing menstrual history, QOL, and symptoms were administered at baseline, day 1 of cycle 4 (or 9 weeks from start of chemotherapy for those who stopped chemotherapy early), and at 6, 12, and 24 months. Seven hundred and eight patients were evaluable for the analysis, with median potential follow-up of 57.5 months. Of these, 321 patients also participated in the QOL substudy. Of the 708 patients, 83% reported ≥1 episode of amenorrhea for ≥6 months. The estimated rate of resumption of menses at 24 months was 45.3% for women <40 years, 10.9% for women 40–50, and 3.2% for women >50 years. Those treated with tamoxifen were more likely to become amenorrheic (p = 0.003). Menstrual status was not significantly associated with QOL or symptoms. Prolonged amenorrhea is associated with a regimen that contains doxorubicin, cyclophosphamide, and docetaxel, and is age dependent and impacted by tamoxifen use. Vasomotor symptoms are common in this patient population but are not associated with menstrual status. These results can be used to inform premenopausal women about the risk and time course of amenorrhea associated with this common adjuvant therapy regimen, along with the effects on symptoms and QOL.


Similar content being viewed by others
References
Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. (2005) Lancet 365:1687–1717
Early Breast Cancer Trialists’ Collaborative Group (1996) Ovarian ablation in early breast cancer: overview of the randomised trials. Lancet 348:1189–1196
Fisher B, Ravdin RG, Ausman RK, Slack NH, Moore GE, Noer RJ (1968) Surgical adjuvant chemotherapy in cancer of the breast: results of a decade of cooperative investigation. Ann Surg 168:337–356
Bonadonna G, Valagussa P, Moliterni A, Zametti M, Brambilla C (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer—the results of 20 years of follow-up. N Engl J Med 332:901–906
Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE (2003) Breast Cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol 21:4184–4193
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G (1997) Reliability and validity of the functional assessment of cancer therapy—breast quality-of-life instrument. J Clin Oncol 15:974–986
Land SR, Kopec JA, Yothers G, Anderson S, Day R, Tang G, Ganz PA, Fisher B, Wolmark N (2004) Health-related quality of life in anxillary node-negative, estrogen receptor-negative breast cancer patients undergoing AC versus CMF chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel Project B-23. Breast Cancer Res Treat 86:153–164
Day R; National Surgical Adjuvant Breast and Bowel Project P-1 Study (NSABP P-1) (2001) Quality of life and tamoxifen in a breast cancer prevention trial: a summary of findings from the NSABP P-1 study. Ann NY Acad Sci 949:143–150
Ganz PA, Day R, Ware JE Jr, Redmond C, Fisher B (1995) Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst 87:1372–1382
Land SR, Wickerham DL, Costantino JP, Ritter MW, Vogel VG, Lee M, Pajon ER, Wade JL III, Dakhil S, Lockhart JB Jr, Wolmark N, Ganz PA (2006) Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2742–2751
Cella D (1996) Manual of the Functional Assessment of Cancer Therapy (FACT) Scales and the Functional Assessment of HIV Infection (FAHI) Scale, version 3. Lippincott-Raven, Philadelphia
Andolsek KM (1992) Cycle control with triphasic norgestimate and ethinyl estradiol, a new oral contraceptive agent. Acta Obstet Gynecol Scand Suppl 156:22–26
Coutinho EM, Spinola P, Barbosa I, Gatto M, Tomaz G, Morais K, Yazlle ME, deSouza RN, Pinho Neto JS, Leal Wde B, Leal C, Hippolito SB, Abranches AD (1997) Multicenter, double-blind, comparative clinical study on the efficacy and acceptability of a monthly injectable contraceptive combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate compared to a monthly injectable contraceptive combination of 90 mg dihydroxyprogesterone acetophenide and 6 mg estradiol enanthate. Contraception 55:175–181
Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, Sukumvanich P (2006) Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol 24:1045–1051
Walshe JM, Denduluri N, Swain SM (2006) Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol 24:5769–5779
Colleoni M, Gelber S, Goldhirsch A, Aebi S, Castiglione-Gertsch M, Price KN, Coates AS, Gelber RD; International Breast Cancer Study Group (2006) Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13–93. J Clin Oncol 24:1332–1341
Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, Tomiak E, Al-Tweigeri T, Chap L, Juhos E et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313
Boccardo F, Rubagotti A, Bruzzi P, Cappellini M, Isola G, Nenci I, Piffanelli A, Scanni A, Sismondi P, Santi L et al (1990) Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node-positive, estrogen receptor-positive breast cancer patients: results of a multicentric Italian study. Breast Cancer Adjuvant Chemo-Hormone Therapy Cooperative Group. J Clin Oncol 8:1310–1320
Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N (1999) Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17:2365–2370
Rose DP (1979) Effect of cytotoxic agents and antihormonal therapy on endocrine function. In: Bulbrook RD, Taylor DJ (eds) Commentaries on Research in Breast Disease. Alan R. Liss, New York
Acknowledgments
Public Health Service grants U10CA-12027, U10CA-37377, U10CA-69651, and U10CA-69974 from the National Cancer Institute, Department of Health and Human Services provided support for this article. The views expressed in this article are solely those of the authors and do not necessarily represent the official views of the National Cancer Institute or the U.S. federal government. We wish to thank Barbara C. Good, PhD, Director of Scientific Publications for the NSABP, for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work represents original research by the authors. Previously presented at ASCO 2005 as a poster (abstr # 537).
Rights and permissions
About this article
Cite this article
Swain, S.M., Land, S.R., Ritter, M.W. et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat 113, 315–320 (2009). https://doi.org/10.1007/s10549-008-9937-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-9937-0