Abstract
Background
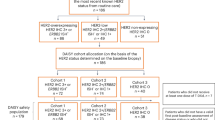
A randomized Phase II study evaluated the activity of weekly paclitaxel versus its combination with trastuzumab for treatment of patients with advanced breast cancer overexpressing HER-2.
Patients and methods
Among 124 patients randomized, 123 are assessable for toxicity and 118 for response. Patients received weekly paclitaxel single agent (80 mg/m2) or combined with trastuzumab (4 mg/kg loading dose, then weekly 2 mg/kg). HER-2 overexpression was determined by immunohistochemistry (IHC). Patients with 2+/3+ IHC scores were eligible. IHC was compared with HER-2 serum extracellular domain (ECD).
Results
Patient characteristics were similar in the two arms. Both treatments were feasible and well tolerated with no grade 4 hematologic toxicity. No patient developed cardiac toxicity. The combined treatment was statistically significant superior for overall response rate (ORR) (75% vs. 56.9%; P = 0.037), particularly in the subset of IHC 3+ patients (84.5% vs. 47.5%; P = 0.00050). A statistically significant better median time to progression was seen in the subgroup with IHC 3+ (369 vs. 272 days; P = 0.030) and visceral disease (301 vs. 183 days; P = 0.0080) treated with combination. Multivariable analysis of predictive factors showed that only IHC score retained statistically significant value for ORR (P = 0.0035).
Conclusion
Weekly paclitaxel plus trastuzumab is highly active and safe and it is superior to paclitaxel alone in patients with IHC score of 3+.
Similar content being viewed by others
References
Ellis MJ, Hayes DF, Lippman ME (2000) Treatment of metastatic breast cancer. In: Harris JR, Lippman ME, Morrow M et al (eds) Diseases of the breast. Lippincott Williams and Wilkins, Philadelphia (PA), pp 749–798
Gasparini G, Longo R, Torino F et al (2005) Therapy of breast cancer with molecular targeting agents. Ann Oncol 16(Suppl 4):28–36
Salomon DS, Brandt R, Fortunato C et al (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Critical Rev Oncol Hematol 19:183–232
Hill CS, Treisman R (1995): Transcriptional regulation by extracellular signals; mechanisms and specificity. Cell 80:199–211
Slamon DJ, Clark GM, Wong SG et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Quenel N, Wafflart J, Borrichon F et al (1995) The prognostic value of c-erbB2 in primary breast carcinomas: a study of 942 cases. Breast Cancer Res Treat 35:283–291
McKeage K, Perry CM (2002) Trastuzumab. Drugs 62:209–243
Pegram MD, Lipton A, Hayes DF et al (1998) Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185 HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J␣Clin Oncol 16:2659–2671
Burstein HJ, Kuter I, Campos SM et al (2001) Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol 19:2722–2730
Seidman AD, Fornier MN, Esteva FJ et al (2001) Weekly trastuzumab and paclitaxel for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 19:2587–2595
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Osoba D, Slamon DJ, Burchmore M et al (2002) Effects on quality of life of combined trastuzumab and chemotherapy in women with metastatic breast cancer. J Clin Oncol 20:3106–3013
Pegram M, Hsu S, Lewis G et al (1999) Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18:2241–2251
Pegram MD, Konecny GE, O’Callaghan C et al (2004) Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 96:739–749
Gori S, Colozza M, Mosconi AM et al (2004) Phase II study of weekly paclitaxel and trastuzumab in anthracycline- and taxane-pretreated patients with HER2-overexpressing metastatic breast cancer. Br J Cancer 90:36–40
Fountzilas G, Tsavdaridis D, Kalogera-Fountzila A et al (2001) Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann Oncol 12:1545–1551
Seidman AD, Hudis CA, Albanel J et al (1998): Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 16:3353–3361
Perez EA, Vogel CL, Irwin DH et al (2001) Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol 19:4216–4223
Seidman AD, Berry D, Cirrincione C et al: CALGB 9840 (2004) Phase III study of weekly (w) paclitaxel (P) via 1-hour(h) infusion versus standard (S) 3h infusion every three week in the treatment of metastatic breast cancer (MBC), with trastuzumab (T) for HER2 positive MBC and randomized for T in HER2 normal MBC. Proc Am Soc Clin Oncol 22:512 (abstr)
Dittadi R, Zancan M, Perasole A et al (2001) Evaluation of HER-2/neu in serum and tissue of primary and metastatic breast cancer patients using an automated enzyme immunoassay. Int J Biol Markers 16:255–261
Moher D, Schulz KF, Altman DG (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 357:1191–1194
Baselga J, Tripathy D, Mendelsohn J et al (1996) Phase II study of weekly intravenous recombinant humanized anti-p185 HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 14:737–744
Bell R, Verma S, Untch M et al (2004) Maximizing clinical benefit with trastuzumab. Semin Oncol 31(Suppl 10):35–44
Jones S, Erban J, Overmoyer B et al (2003) Randomized trial comparing docetaxel and paclitaxel in patients with metastatic breast cancer. Breast Cancer Res Treat 82:10(abstr)
Marty M, Cognetti F, Maraninchi D et al (2005) Randomized Phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 Study Group. J Clin Oncol 23:4265–4274
Pegram MD, Pienkowski T, Northfelt DW et al (2004) Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst 96:759–769
Baselga J, Climent MA, Lluch A et al (2004) Results of a phase II study of liposomal doxorubicin (Myocet) in combination with weekly paclitaxel and trastuzumab (Herceptin) in patients with HER2-positive locally advanced or metastatic breast cancer (LA/MBC). Eur J Cancer 2:262 (abstr)
Esteva FJ, Valero V, Booser D et al (2002) Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 20:1800–08
Tedesco KL, Thor AD, Johnson DH et al (2004): Docetaxel combined with trastuzumab is an active regimen in HER-2 3+ overexpressing and fluorescent in situ hybridization-positive metastatic breast cancer: a multi-institutional Phase II trial. J Clin Oncol 22:1071–1077
Bocci G, Nicolaou KC, Kerbel RS (2002) Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res 62:6938–6943
Perez EA, Pusztai L, van De Vijver (2004) Improving patient care through molecular diagnostics. Sem Oncol 31(Suppl 10):14–20
Fornier MN, Seidman AD, Schwartz MK et al (2005) Serum HER2 extracellular domain in metastatic breast cancer patients treated with weekly trastuzumab and paclitaxel: association with HER2 status by immunohistochemistry and fluorescence in situ hybridization and with response rate. Ann Oncol 16:234–239
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gasparini, G., Gion, M., Mariani, L. et al. Randomized Phase II Trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res Treat 101, 355–365 (2007). https://doi.org/10.1007/s10549-006-9306-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9306-9