Abstract
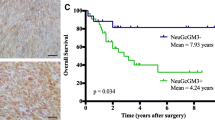
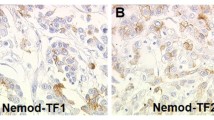
The relevance of certain gangliosides in tumour growth and metastatic dissemination has been well documented, reasons for considering these molecules as potential targets for cancer immunotherapy and diagnosis. GM3(NeuGc) ganglioside is particularly interesting due to its restrictive expression in normal human tissues according to immunohistochemical studies, using either polyclonal or monoclonal antibodies. But both immunohistochemical and biochemical methods have strongly suggested its over-expression in human breast tumours. Nevertheless, the lack of a direct evidence of this antigenic display in human breast cancer has kept the subject controversial. For the first time, we described herein the “in vivo” detection of GM3(NeuGc) ganglioside in human breast primary tumours using a radioimmunoscintigraphic technique with 14F7, a highly specific anti-GM3(NeuGc) ganglioside monoclonal antibody, labelled with 99mTc. In an open, prospective Phase I/II clinical trial, including women diagnosed in stage II breast cancer, the 14F7 monoclonal antibody accumulation in tumours at doses of 0.3 (n=5), 1 (n=5) and 3 mg (n=4) was evaluated. Noteworthy, the immunoscintigraphic study showed antibody accumulation in 100% of patients’ tumours for the 1 mg dose group. In turn, the radioimmunoconjugate injected at doses of 0.3 mg or 3 mg of the antibody, was uptaken by 60 and 33.3% of breast tumours, respectively. “In vivo” immune recognition of GM3(NeuGc) in breast tumours reinforces the value of this peculiar target for cancer immunotherapy.
Similar content being viewed by others
References.
Hougton AN, Mintzer D, Cordon-Cardo C, Welt S, Fliegel B, Vadhan S, Carswell E, Melamed MR, Oettgen HF, Old LJ, 1985 Mouse monoclonal antibody IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanomaProc Natl Acad Sci USA 82: 1242–1246
Zhang S, Cordon Cardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, Lloyd KO, Livingston PO, 1997 Selection of carbohydrate tumour antigens as targets for immune attack using immunohisto-chemistry. I. Focus on gangliosidesInt J Cancer 73: 42–49
Stults CLM, Sweeley CC, Matcher BA, 1989 Glycosphingolipids: structure, biological source, and properties Methods Enzymol 179: 167–214
Hakomori SH, 1991 Possible functions of tumour-associated carbohydrate antigens Curr Opin Immunol 3: 646–653
Corfield AP, Schauer R, 1982 Occurrence of sialic acidsCell Biol Monogr 10: 5–50
Leeden RW, Yu RK, 1976 Chemistry and analysis of sialic acid In: Rosemberg A, Schengtrund CL, (eds). Biological Role of Sialic Acid Plenum New York pp. 1–48
Kawai T, Kato A, Higashi H, Kato S, Naiki M, 1991 Quantitative determination of N glycolylneuraminic acid expression in human cancerous tissues and avian lymphoma cell lines as a tumour-associated sialic acid by gas chromatography–mass spectometryCancer Res 51: 1242–1246
Higashi H, Sasabe T, Fukui Y, Maru M, Kato S, 1988 Detection of gangliosides as N-glycolylneuraminic acid-specific tumour-associated Hanganutziu–Deicher antigen in human retinoblastoma cellsJpn J Cancer Res 79: 952–956
Fukui Y, Maru M, Ohkawara KI, Miyake T, Osada Y, Wang D, Ito T, Higashi H, Naiki M, Wakamiya N, Kato S, 1989 Detection of glycoproteins as tumour-associated Hanganutziu–Deicher antigen in human gastric cancer cell line, NUGC4 Biochem Biophys Res Commun 160: 1149–1154
Koda T, Shimosakoda T, Asaoka H, Nishinaka S, Tamura I, Nakaba H, Matsuda H, 1994 Detection of the Hanganutziu–Deicher antigen in patients with hepatocellular carcinomaInt Hepatol Commun 2:310–315
Furukawa K, Yamaguchi H, Oettgen HF, Old LJ, Lloyd KO, 1988 Analysis of the expression of N-glycolylneuraminic acid-containing gangliosides in cell and tissues using two human monoclonal antibodiesJ Biol Chem 263:18507–18512
Miyake M, Hashimoto K, Ito M, Ogawa O, Arai E, Hitomi S, Kannagi R, 1990 The abnormal occurrence and the differentiation-dependent distribution of N-acetyl and N-glycolyl species of the ganglioside GM2 in human germ cell tumours. A study with specific monoclonal antibodiesCancer 65: 499–505
Marquina G, Waki H, Fernández LE, Kon K, Carr A, Valiente O, Pérez R, Ando S, 1996 Gangliosides expressed in human breast cancerCancer Res 56: 5165–5171
Carr A, Mullet A, Mazorra Z, Vázquez AM, Alfonso M, Mesa C, Rengifo E, Pérez R, Fernández LE, 2000 A mouse IgG1 monoclonal antibody specific for N-Glycolyl GM3 ganglioside recognized breast and melanoma tumours Hybridoma 19:241–247
Carr A, Mesa C, Arango M, Vázquez AM, Fernández LE, 2002 “In vivo” and “in vitro” anti-tumour effect of 14F7 monoclonal antibody Hybridoma and Hybridomics 21: 463–468
Mather JS, Ellison D, 1990 Reduction-mediated technetium-99 m labeling of monoclonal antibodiesJ Nucl Med 31: 692–697
Hsu SM, Raine L, Fanger H, 1989 Use the avidin–biotin-peroxidase complex (ABC) in immunoperoxidase technique: a comparison between ABC and unlabelled antibody PAP proceduresJ Histochem Cytochem 29:577–580
American Cancer Society. Cancer Facts and Figures 2004. Atlanta, Ga: American Cancer Society, 2004. tttp://www.cancer. org/docroot/STT/stt_O.asp
Hakomori S, 1985 Aberrant glycosylation in cancer cell membranes as focused on glycolipids: Overview and perperstiveCancer Res 45:2405–2414
Plalet N, Cathiard AM, Gleizes M, Garcia M, 2004 Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasionCrit Rev Oncol Hematol 51: 55–67
Revillion F, Bonneterre J, Peyrat JP, 1998 ERBB2 oncogene in human breast cancer and its clinical significanceEur J Cancer 34: 791–808
Sharma AK, Horgan K, Douglas-Jones A, McClelland R, Gee J, Nicholson R, 1994 Dual immunocytochemical analysis of oestrogen and epidermal growth factor receptors in human breast cancerBr J Cancer 69: 1032–1037
Nicholson RI, Hutcheson IR, Harper ME, Knowlden JM, Barrow D, McClelland RA, Jones HE, Wakeling AE, Gee JM, 2001 Modulation of epidermal growth factor receptor in endocrine resistant, oestrogen receptor-positive breast cancerEndcr Relat Cancer 8: 175–182
Estevez F, Carr A, Solarzano L, Valiente O, Mesa C, Barroso O, Sierra G, Fernandez LE, 1999 Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP)Vaccine 18: 190–197
Bitton RJ, Guthmann MD, Gabri MR, Carnero AJ, Alonso DF, Fainboim L, Gomez DE, 2002 Cancer vaccines: An update with special focus on ganglioside antigens (Review) Oncol Reports 9:267–276
Carr A, Rodriguez E, Arango M, Camacho R, Osorio M, Gabri M, Carrillo G, Valdes Z, Bebelagua Y, Perez R, Fernandez LE, 2003 Immunotherapy of advanced breast cancer with a heterophilic ganglioside (NeuGcGM3) cancer vaccine J Clin Oncol 21:1015–1021
Fernández LE, Alonso DF, Gómez DE, Vázquez AM, 2003 Ganglioside-based vaccines and anti-idiotype antibodies for active immunotherapy against cancerExprt Rev Vaccines 2: 89–95
Mesa C, León J, Rigley K, Fernández LE, 2004 Very small proteoliposomes derived from Neisseria meningitides: an effective adjuvant for Th1 induction and dendritic cell activationVaccine 22:3045–3052
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliva, J.P., Valdés, Z., Casacó, A. et al. Clinical evidences of GM3 (NeuGc) ganglioside expression in human breast cancer using the 14F7 monoclonal antibody labelled with 99mTc. Breast Cancer Res Treat 96, 115–121 (2006). https://doi.org/10.1007/s10549-005-9064-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-005-9064-0