Abstract
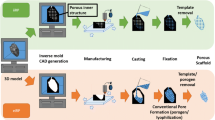
Advanced scaffold fabrication techniques such as Rapid Prototyping (RP) are generally recognized to be advantageous over conventional fabrication methods in terms architectural control and reproducibility. Yet, most RP techniques tend to suffer from resolution limitations which result in scaffolds with uncontrollable, random-size pores and low porosity, albeit having interconnected channels which is characteristically present in most RP scaffolds. With the increasing number of studies demonstrating the profound influences of scaffold pore architecture on cell behavior and overall tissue growth, a scaffold fabrication method with sufficient architectural control becomes imperative. The present study demonstrates the use of RP fabrication techniques to create scaffolds having interconnected channels as well as controllable micro-size pores. Adopted from the concepts of porogen leaching and indirect RP techniques, the proposed fabrication method uses monodisperse microspheres to create an ordered, hexagonal closed packed (HCP) array of micro-pores that surrounds the existing channels of the RP scaffold. The pore structure of the scaffold is shaped using a single sacrificial construct which comprises the microspheres and a dissolvable RP mold that were sintered together. As such, the size of pores as well as the channel configuration of the scaffold can be tailored based on the design of the RP mold and the size of microspheres used. The fabrication method developed in this work can be a promising alternative way of preparing scaffolds with customized pore structures that may be required for specific studies concerning cell-scaffold interactions.









Similar content being viewed by others
References
C.J. Bettinger, J.T. Borenstein, R. Langer, Microfabrication techniques in scaffold development, in Nanotechnology and tissue engineering: the scaffold, ed. by C.T. Laurencin, L.S. Nair (CRC press, Boca Raton, 2008), p. 359
A. Bozkurt, G.A. Brook, S. Moellers, F. Lassner, B. Sellhaus, J. Weis, M. Woeltje, N. Pallua, In vitro assessment of axonal growth using dorsal root ganglia explants in a novel three-dimensional collagen matrix. Tissue Eng 13, 2971–2979 (2007)
C.T. Buckley and K.U. O’Kelly, Regular scaffold fabrication techniques for investigations in tissue engineering, in Topics in Bio-Mechanical Engineering, ed. by P.J. Predergast and P.E. McHugh (TCBE & NCBES, 2004), p. 147–166
E.P.A. Caren, W.S. Karim, L.L. Ann, B.R. Dave, S.T. Sunil, C.O. Roy, A.B. Edward, Comparative effects of scaffold pore size, pore volume, and total void volume on cranial bone healing patterns using microsphere-based scaffolds. J Biomed Mater Res A 89, 632–641 (2009)
L. Draghi, S. Resta, M.G. Pirozzolo, M.C. Tanzi, Microspheres leaching for scaffold porosity control. J Mater Sci Mater Med 16, 1093–1097 (2005)
B. Duan, W.L. Cheung, M. Wang, Optimized fabrication of Ca-P/PHBV nanocomposite scaffolds via selective laser sintering for bone tissue engineering. Biofabrication 3, 015001 (2011)
J.C.Y. Dunn, Analyses of cell growth in tissue-engineering scaffolds. Regen Med 3, 421–424 (2008)
R.C. Dutta, A.K. Dutta, Cell-interactive 3D-scaffold; advances and applications. Biotechnol Adv 27, 334–339 (2009)
A.J. Engler, S. Sen, H.L. Sweeney, D.E. Discher, Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006)
L.E. Freed, G. Vunjaknovakovic, R.J. Biron, D.B. Eagles, D.C. Lesnoy, S.K. Barlow, R. Langer, Biodegradable polymer scaffolds for tissue engineering. Biotechnology (NY) 12, 689–693 (1994)
L.G. Griffith, Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann New York Acad Sci 961, 83–95 (2002)
J.J. Guan, J.J. Stankus, W.R. Wagner, Development of composite porous scaffolds based on collagen and biodegradable poly(ester urethane)urea. Cell Transplant 15, S17–S27 (2006)
Y.Y. Hsu, J.D. Gresser, D.J. Trantolo, C.M. Lyons, P.R. Gangadharam, D.L. Wise, Effect of polymer foam morphology and density on kinetics of in vitro controlled release of isoniazid from compressed foam matrices. J Biomed Mater Res 35, 107–116 (1997)
D.W. Hutmacher, Scaffolds in tissue engineering bone and cartilage. Biomaterials 21, 2529–2543 (2000)
S.J. Kalita, S. Bose, H.L. Hosick, A. Bandyopadhyay, Development of controlled porosity polymer-ceramic composite scaffolds via fused deposition modeling. Mater Sci Eng C 23, 611–620 (2003)
H.-W. Kang, J.-W. Rhie, D.-W. Cho, Development of a bi-pore scaffold using indirect solid freeform fabrication based on microstereolithography technology. Microelectronic Eng 86, 941–944 (2009)
T.S. Karande, J.L. Ong, C.M. Agrawal, Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, architecture, and nutrient mixing. Ann Biomed Eng 32, 1728–1743 (2004)
S.S. Kim, H. Utsunomiya, J.A. Koshi, B.M. Wu, M.J. Cima, J. Sohn, K. Mukai, L.G. Griffith, J.P. Vacanti, Survival and function of hepatocytes on a novel three-dimensional synthetic biodegradable polymer scaffold with an intrinsic network of channels. Ann Surg 228, 8–13 (1998)
K.F. Leong, C.M. Cheah, C.K. Chua, Solid freeform fabrication of three dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 24, 2363–2378 (2003)
K.F. Leong, C.K. Chua, N. Sudarmadji, W.Y. Yeong, Engineering functionally graded tissue engineering scaffolds. J Mech Behav Biomed Mater 1, 140–152 (2008)
M.F. Leong, M.Z. Rasheed, T.C. Lim, K.S. Chian, In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly(D, L-lactide) scaffold fabricated by cryogenic electrospinning technique. J Biomed Mater Res A 91, 231–240 (2009)
S.M. Lien, L.Y. Ko, T.J. Huang, Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater 5, 670–679 (2009)
P.X. Ma, Scaffolds for tissue fabrication. Mater Today 7, 30–40 (2004)
I. Martin, D. Wendt, M. Heberer, The role of bio-reactors in tissue engineering. Trends Biotechnol 22, 80–86 (2004)
A.G. Mikos, A.J. Thorsen, L.A. Czerwonka, Y. Bao, R. Langer, D.N. Winslow, J.P. Vacanti, Preparation and characterization of poly(L-Lactic Acid) foams. Polymer 35, 1068–1077 (1994)
D.J. Mooney, D.F. Baldwin, N.P. Suh, J.P. Vacanti, R. Langer, Novel approach to fabricate porous sponges of poly(D, L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 17, 1417–1422 (1996)
M.J. Moore, J.A. Friedman, E.B. Lewellyn, S.M. Mantila, A.J. Krych, S. Ameenuddin, A.M. Knight, L. Lu, B.L. Currier, R.J. Spinner, R.W. Marsh, A.J. Windebank, M.J. Yaszemski, Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials 27, 419–429 (2006)
D. Muller, H. Chim, A. Bader, M. Whiteman, J. Schantz, Vascular guidance: microstructural scaffold patterning for inductive neovascularization. Stem Cells Int 2011, 1–6 (2011)
M. Oates, R. Chen, M. Duncan, J. Hunt, The angiogenic potential of three-dimensional open porous synthetic matrix materials. Biomaterials 28, 3679–3686 (2007)
S.H. Oh, I.K. Park, J.M. Kim, J.H. Lee, In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 28, 1664–1671 (2007)
G. Papavasiliou, M.H. Cheng, E.M. Brey, Strategies for vascularization of polymer scaffolds. J Investig Med 58, 838–844 (2010)
B.D. Ratner, A paradigm shift: biomaterials that heal. Polymer Int 56, 1183–1185 (2007)
T. Sabia, R. Krishnendu, Influence of scaffold physical properties and stromal cell coculture on hematopoietic differentiation of mouse embryonic stem cells. Biomaterials 27, 6024–6031 (2006)
E. Sachlos, J.T. Czernuszka, Making tissue engineering scaffolds work. Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater J 5, 29–40 (2003)
E. Sachlos, N. Reis, C. Ainsley, B. Derby, J.T. Czernuszka, Novel collagen scaffolds with predefined internal morphology made by solid freeform fabrication. Biomaterials 24, 1487–1497 (2003)
S. Singare, S.Y. Zhong, Z.Z. Sun, A method to fabricate liver tissue engineering scaffold. Journal of Biomimetics, Biomaterials & Tissue Engineering 11, 73–80 (2011)
J.M. Taboas, R.D. Maddox, P.H. Krebsbach, S.J. Hollister, Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials 24, 181–194 (2003)
P. Tayalia, C.R. Mendonca, T. Baldacchini, D.J. Mooney, E. Mazur, 3D cell migration studies using two-photon engineered polymer scaffolds. Adv Mater 20, 4494–4498 (2008)
L.C. Wang, J.W. Chen, H.L. Liu, Z.Q. Chen, Y. Zhang, C.Y. Wang, Z. Feng, Synthesis and evaluation of biodegradable segmented multiblock poly(ether ester) copolymers for biomaterial applications. Polymer Int 53, 2145–2154 (2004)
K. Whang, C. Thomas, K. Healy, G. Nuber, A novel method to fabricate bioabsorbable scaffolds. Polymer 36, 837–842 (1995)
F.E. Wiria, K.F. Leong, C.K. Chua, Y. Liu, Polycaprolactone/Hydroxyapatite for tissue engineering scaffold fabriaction using selective laser sintering. Acta Biomater 3, 1–12 (2007)
W.Y. Yeong, C.K. Chua, K.F. Leong, M. Chandrasekaran, Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol 22, 643–652 (2004)
W.Y. Yeong, C.K. Chua, K.F. Leong, Indirect fabrication of collagen scaffold based on inkjet printing technique. Rapid Prototyping J 12, 229–237 (2006)
J. Zeltinger, J.K. Sherwood, D.A. Graham, R. Mueller, L.G. Griffith, Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng 7, 557–572 (2001)
Y. Zhang, W. Fan, Z. Ma, C. Wu, W. Fang, G. Liu, Y. Xiao. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomaterialia (2010), Ahead of print
W.Y. Zhou, S.H. Lee, M. Wang, W.L. Cheung, Selective laser sintering of tissue engineering scaffolds using poly(l-lactide) microspheres. Key Eng Mater 334–335, 1225–1228 (2007)
Acknowledgement
The authors gratefully acknowledge the financial support of both the Singapore Agency for Science, Technology and Research (ASTAR) through the Singapore-University of Washington Alliance (SUWA) programme and Nanyang Technological University (NTU).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Illustration on the alignment of monodispersed microspheres into a hexagonal arrangement under ultrasound agitation (WMV 1786 kb)
The alignment of monodispersed microspheres under ultrasound agitation showing multilayer stacking of microspheres (WMV 8814 kb)
Rights and permissions
About this article
Cite this article
Tan, J.Y., Chua, C.K. & Leong, K.F. Fabrication of channeled scaffolds with ordered array of micro-pores through microsphere leaching and indirect Rapid Prototyping technique. Biomed Microdevices 15, 83–96 (2013). https://doi.org/10.1007/s10544-012-9690-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-012-9690-3