Abstract
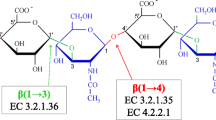
A low molecular weight isoform of hyaluronidase (NNH2) has been isolated from Indian cobra (Naja naja) venom by successive chromatography on Sephadex G-75 and CM-Sephadex C-25 columns. The apparent molecular weight determined by SDS-PAGE is 52 kD, and the pI value is 9.7. NNH2 is an endoglycosidase and exhibits in vitro absolute specificity for hyaluronan; it also hydrolyzed hyaluronan in human skin sections. NNH2 is nontoxic, but it indirectly potentiates the hemorrhagic activity of hemorrhagic complex-I. Curcumin, indomethacin, and tannic acid inhibited dose dependently the degradation of hyaluronan by NNH2.
Similar content being viewed by others
REFERENCES
Warrell, D. A. (1996) in Textbook of Medicine (Wealtherall, D. J., Ledingham, J. G. G., and Warrell, D. A., eds.) Oxford University Press, Oxford.
Chippaux, J. P. (1998) Bull. WHO, 76, 515–524.
Kini, R. M. (1997) Venom Phospholipase A 2 Enzymes: Structure, Function and Mechanism, J. Wiley, New York.
Kini, R. M. (1998) Toxicon, 36, 1659–1670.
Harvey, A. L., Bradley, K. N., Cocharan, S. A., Rowan, E. G., Pratt, J. A., Quillfeldt, J. A., and Jerusalinsky, D. A. (1998) Toxicon, 36, 1635–1640.
Markland, F. S. (1998) Toxicon, 36, 1749–1800.
Shashidhara murthy, R., Jagadeesha, D. K., Girish, K. S., and Kemparaju, K. (2002) Mol. Cell. Biochem., 229, 93–101.
Gutierrez, J. M., and Ownby, C. L. (2003) Toxicon, 42, 915–931.
Teixeira, C. F. P., Landucci, E. C. T., Antunes, E., Chacur, M., and Cury, Y. (2003) Toxicon, 42, 947–962.
Gopalakrishnakone, P., Ponraj, D., and Thwin, M. M. (1997) in Venom Phospholipase A 2 Enzymes: Structure, Function and Mechanism (Kini, R. M., ed.) John Wiley & Sons Ltd., pp. 287–319.
Gutierrez, J. M., and Rucavado, A. (2000) Biochimie, 82, 841–850.
Girish, K. S., Jagadeesha, D. K., Rajeev, K. B., and Kemparaju, K. (2002) Mol. Cell. Biochem., 40, 105–110.
Anai, K., Sugiki, M., Yoshida, E., and Maruyama, M. (2002) Toxicon, 40, 63–68.
Girish, K. S., Shashidhara murthy, R., Nagaraju, S., Gowda, T. V., and Kemparaju, K. (2004) Biochimie, 86, 193–202.
Maruyama, M., Sugiki, M., Yoshida, E., Mihara, H., and Nakajima, N. (1992) Toxicon, 30, 853–864.
Estevao-Costa, M. I., Diniz, C. R., Magalhaes, A., Markland, F. S., and Sanchez, E. F. (2000) Thromb. Res., 99, 363–376.
Leon, G., Valverde, J. M., Rojas, G., Lomonte, B., and Gutierrez, J. M. (2000) Toxicon, 38, 233–244.
Yingprasertchai, S., Bunyasrisawt, S., and Ratanabanangkoon, K. (2003) Toxicon, 42, 635–646.
Tu, A. T., and Hendon, R. R. (1983) Comp. Biochem. Physiol., 76, 337–383.
Stern, M., and Stern, R. (1992) Matrix, 12, 397–403.
Laemmli, U. K. (1970) Nature, 227, 680–685.
Fiszer-szafarz, B. (1984) Analyt. Biochem., 143, 76–81.
Cevellos, M. A., Navarro-Duque, C., Varela-Julia, M., and Alagon, A. C. (1992) Toxicon, 30, 925–930.
Menzel, E. J., and Farr, C. (1998) Cancer Lett., 131, 3–11.
Uma, B. (1999) Ph. D. Thesis, University of Mysore, Mysore, India.
Kondo, H., Kondo, S., Ikezawa, H., Muruta, R., and Ohsaka, A. (1960) Jpn. J. Med. Sci. Biol., 13, 43–51.
Tammi, R., Ripellino, J. A., Margolis, R. U., and Tammi, M. (1988) J. Invest. Dermatol., 90, 412–414.
Meyer, L. J. M., and Stern, R. (1994) J. Invest. Dermatol., 102, 385–389.
Rucavado, A., Escalante, T., Franceschi, A., Chaves, F., Leon, G., Cury, Y., Ovadia, M., and Gutierrez, J. M. (2000) Am. J. Trop. Med. Hyg., 63, 313–319.
Escalante, T., Franceschi, A., Rucavado, A., and Gutierrez, J. M. (2000) Biochem. Pharmacol., 60, 269–274.
Leon, G., Rojas, G., Lomonte, B., and Gutierrez, J. M. (1997) Toxicon, 35, 1627–1637.
Gutierrez, J. M., Chaves, F., Rojas, G., Bogarin, G., and Lomonte, B. (1996) in Envenomings and Their Treatments (Bon, C., and Goyffon, M., eds.) Foundation Marcel Merieux Luyon, Marcel, pp. 223–231.
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Biokhimiya, Vol. 70, No. 6, 2005, pp. 855–860.
Original Russian Text Copyright © 2005 by Girish,, Kemparaju.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM04-224, October 24, 2004.
Rights and permissions
About this article
Cite this article
Girish, K.S., Kemparaju, K. A Low Molecular Weight Isoform of Hyaluronidase: Purification from Indian Cobra (Naja naja) Venom and Partial Characterization. Biochemistry (Moscow) 70, 708–712 (2005). https://doi.org/10.1007/s10541-005-0172-6
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s10541-005-0172-6