Abstract
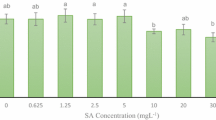
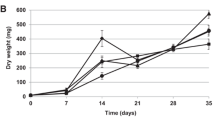
The effect of salicylic acid on the content of soluble proteins and individual polypeptides in Tatar buckwheat Fagopyrum tataricum calluses differing in ability for morphogenesis was studied. Changes in the protein composition of the calluses cultivated in the dark and in the light indicated the higher sensitivity of the non-morphogenic callus. Different response of callus cultures to salicylic acid and conditions of cultivation (light, darkness) is suggested to be associated with the antioxidant defense system, which is, in particular, characterized by the hydrogen peroxide content in the calluses. Salicylic acid increased the H2O2 content in non-morphogenic calluses more strongly than in morphogenic calluses, and the difference was more significant for the calluses cultivated in the light.
Similar content being viewed by others
REFERENCES
Raskin, I. (1992) Annu. Rev. Plant Physiol. Plant Mol. Biol., 43, 439–463.
Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., Ward, E., Kessmann, H., and Ryals, J. (1993) Science, 261, 754–756.
Ryals, J. A., Neuenschwander, U. H., Willits, M. G., Molina, A., Steiner, H.-Y., and Hunt, M. D. (1996) Plant Cell, 8, 1809–1819.
Durner, J., Shah, J., and Klessig, D. I. (1997) Trends Plant Sci., 2, 266–374.
Malamy, J., Carr, J. P., Klessig, D. F., and Raskin, I. (1990) Science, 250, 1002–1004.
Yalpani, N., Leon, J., Lawton, M. A., and Raskin, I. (1993) Plant Physiol., 103, 315–321.
Leon, J., Lawton, M. A., and Raskin, I. (1995) Plant Physiol., 108, 1673–1678.
Chen, Z., Silva, H., and Klessig, D. F. (1993) Science, 262, 1883–1886.
Rao, M. V., Paliyath, G., Ormrod, D. P., Murr, D. P., and Watkins, C. B. (1997) Plant Physiol., 115, 137–149.
Saprin, A. N., and Kalinina, E. V. (1999) Usp. Biol. Khim., 39, 289–326.
Bowler, C., van Montagu, M., and Inze, D. (1992) Annu. Rev. Plant Physiol. Plant Mol. Biol., 43, 83–116.
Hernandez, J. A., Corpas, F. J., Gomez, M., del Rio, L. A., and Sevilla, F. (1993) Physiol. Plant., 89, 103–110.
Antoniw, J. F., and White, R. F. (1980) Phytopathol. Z., 98, 331–341.
Delaney, T. P., Uknes, S., Vernooij, B., Friedrich, L., Weymann, K., Nwgrotto, D., Gaffney, T., Gut-Rella, M., Kessmann, H., and Ward, E. (1994) Science, 266, 1247–1250.
Rumyantseva, N. I., Valieva, A. I., Samokhvalova, N. A., Mukhitov, A. R., Ageeva, M. V., and Lozovaya, V. V. (1998) Tsitologiya, 40, 835–843.
Gamborg, O. L., Miller, R. A., and Ojima, K. (1968) Exp. Cell Res., 50, 151–158.
Rumyantseva, N. I., Sal’nikov, V. V., Fedoseeva, N. V., and Lozovaya, V. V. (1992) Fiziol. Rast., 39, 143–151.
Bradford, M. M. (1976) Analyt. Biochem., 72, 248–254.
Laemmli, U. K. (1970) Nature, 227, 680–585.
Weber, K., and Osborn, M. (1969) J. Biol. Chem., 16, 4406–4412.
Bellincampi, D., Dipierro, N., Salvi, G., Gervone, F., and Lorenzo, C. D. (2000) Plant Physiol., 122, 1379–1385.
Ramanujam, M. P., Abduljaleel, V., and Kumaravelu, G. (1998) Biol. Plant., 41, 307–311.
Asthana, J. S., and Srivastava, H. S. (1978) Indian J. Plant Physiol., 21, 150–155.
Ellis, R. J., Gallagher, T. F., Jenkins, G. I., and Lennox, C. R. (1984) J. Embryol. Exp. Morphol., 83, 163–178.
Portis, A. R. J. (1992) Annu. Rev. Plant Physiol. Plant Mol. Biol., 43, 415–437.
Chang, C. J., and Kao, C. H. (1998) Plant Growth Regul., 25, 11–15.
Robinson, S. P., and Portis, A. R. (1988) Plant Physiol., 86, 293–298.
Campbell, W. J., and Orgen, W. L. (1990) Plant Physiol., 92, 110–115.
Thompson, W. F., and White, M. J. (1991) Annu. Rev. Plant Physiol. Plant Mol. Biol., 42, 423–466.
Kefeli, V. I. (1975) in Photoregulation of Plant Metabolism and Morphogenesis (Kursanov, A. L., and Voskresenskaya, N. P., eds.) [in Russian], Nauka, Moscow, pp. 209–227.
Genoud, T., Buchala, A. J., Chua, N.-H., and Metraux, J.-P. (2002) Plant J., 31, 87–95.
Ruffer, M., Steipe, B., and Zenk, M. H. (1995) FEBS Lett., 377, 175–180.
Ganesan, V., and Thomas, G. (2001) Plant Sci., 160, 1095–1106.
Sutherland, M. W. (1991) Physiol. Mol. Plant Pathol., 39, 79–93.
Luo, J.-P., Jiang, S.-T., and Pan, L.-J. (2001) Plant Sci., 161, 125–132.
Lin, C. L., and Kao, C. H. (2000) Plant Growth Regul., 30, 151–155.
Bernal, M. A., Bisbis, B., Pedreno, M. A., Kevars, C., Penel, C., and Gaspar, T. (1997) Biol. Plant., 39, 161–168.
Repka, V., and Fischerova, I. (1997/98) Biol. Plant., 40, 605–615.
Morimura, Y., Iwamoto, K., Ohya, T., Igarashi, T., Nakamura, Y., Kubo, A., Tanaka, K., and Ikawa, T. (1999) Plant Sci., 142, 123–132.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Biokhimiya, Vol. 70, No. 3, 2005, pp. 390–396.
Original Russian Text Copyright © 2005 by Maksyutova, Galeeva, Rumyantseva, Viktorova.
Rights and permissions
About this article
Cite this article
Maksyutova, N.N., Galeeva, E.I., Rumyantseva, N.I. et al. Effect of salicylic acid on protein composition of Tatar buckwheat Fagopyrum tataricum calluses with different ability for morphogenesis. Biochemistry (Moscow) 70, 316–321 (2005). https://doi.org/10.1007/s10541-005-0117-0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s10541-005-0117-0