Abstract
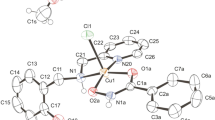
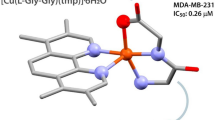
In this study, the N,N,O metal chelator 2-pyridinecarboxaldehydeisonicotinoyl hydrazone (HPCIH, 1) and its derivatives 2-acetylpyridine-(HAPIH 2), 2-pyridineformamide-(HPAmIH, 3) and pyrazineformamide-(HPzAmIH, 4) were employed in the synthesis of four copper(II) complexes, [Cu(HPCIH)Cl2]·0.4H2O (5), [Cu(HAPIH)Cl2]·1.25H2O (6), [Cu(HPAmIH)Cl2]·H2O (7) and [Cu(HPzAmIH)Cl2]·1.25H2O (8). The compounds were assayed for their action toward Mycobacterium tuberculosis H37Rv ATCC 27294 strain and the human tumor cell lines OVCAR-8 (ovarian cancer), SF-295 (glioblastoma multiforme) and HCT-116 (colon adenocarcinoma). All copper(II) complexes were more effective in reducing growth of HCT-116 and SF-295 cells than the respective free hydrazones at 5 µg/mL, whereas only complex 7 was more cytotoxic toward OVCAR-8 lines than its ligand HPAmIH. 6 proved to be cytotoxic at submicromolar doses, whose IC50 values (0.39–0.86 µM) are similar to those ones found for doxorubicin (0.23–0.43 µM). Complexes 5 and 6 displayed high activity against M. tuberculosis (MIC = 0.85 and 1.58 µM, respectively), as compared with isoniazid (MIC = 2.27 µM), which suggests the compounds are attractive candidates as antitubercular drugs.




Similar content being viewed by others
References
Ababei LV, Kriza A, Musuc AM, Andronescu C, Rogozea EA (2010) Thermal behavior and spectroscopic studies of complexes of some divalent transitional metals with 2-benzoil pyridilizonicotinoylhydrazone. J Therm Anal Calorim 101:987–996
Addison AW, Rao TN, Reedijk J, Rijin J, Verschoo GC (1984) Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the Crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2’-yl)-2,6-dithiaheptane]copper(II)perchlorate. J Chem Soc, Dalton Trans 7(7):1349–1356
Amim RS, Firmino GSS, Rego ACPD, Nery AL, Da-Silva SAG, Souza MVN, Pessoa C, Resende JALC, Figueroa-Villar JD, Lessa JA (2016) Cytotoxicity and leishmanicidal activity of isoniazid-derived hydrazones and 2-pyrazineformamide thiosemicarbazones. J Braz Chem Soc 27:769–777
Armstrong CM, Bernhardt PV, Chin P, Richardson DR (2003) Structural variations and formation constants of first-row transition metal complexes of biologically active aroylhydrazones. Eur J Inorg Chem 6:1145–1156
Becker E, Richardson DR (1999) Development of novel aroylhydrazone ligands for iron chelation therapy: 2-Pyridylcarboxaldehyde isonicotinoyl hydrazone analogs. J Lab Clin Med 134:510–521
Beraldo H, Gambino D (2004) The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini-Rev Med Chem 4:31–39
Bernardes-Génisson V, Deraeve C, Chollet A, Bernadou J, Pratviel G (2013) Isoniazid: an update on the multiple mechanisms for a singular action. Curr Med Chem 20(35):4370–4385
Bhattacharyya S, Kumar SB, Dutta SK, Tiekink ERT, Chaudhury M (1996) Zinc(II) and copper(II) complexes of pentacoordinating (N4S) ligands with flexible pyrazolyl arms: syntheses, structure, and redox and spectroscopic properties. Inorg Chem 35:1967–1973
Bruker (2014) APEX2, SAINT and SADABS. Bruker AXS Inc, Madison, Wisconsin
Chang H, Jia L, Xu J, Xu Z, Chen R, Wu W, Bie H, Zhu T, Ma T, Wang Y (2015) Syntheses, characterizations, antitumor activities and cell apoptosis induction of Cu(II), Zn(II) and Cd(II) complexes with hydrazone Schiff base derived from isonicotinohydrazide. Inorg Chem Comm 57:8–10
Chaves JDS, Damasceno JL, Paula MCF, de Oliveira PF, Azevedo GC, Matos RC, Lourenço MCS, Tavares DC, Silva H, Fontes APS, de Almeida MV (2015) Synthesis, characterization, cytotoxic and antitubercular activities of new gold(I) and gold(III) complexes containing ligands derived from carbohydrates. Biometals 28:845–860
Cohen MD, Flavian S (1967) Topochemistry. Part XXVI. The absorption spectra of some thermochromic N-salicylideneanilines and hydroxynaphthylideneanilines in the crystal. J Chem Soc B 317:329–334
Despaigne AAR, Da Costa FB, Piro OE, Castellano EE, Louro SRW, Beraldo H (2012) Complexation of 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones to copper(II) as an effective strategy for antimicrobial activity improvement. Polyhedron 38:285–290
Dheda K, Barry CE 3rd, Maartens G (2016) Tuberculosis. Lancet 387:1211–1226
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst 42:339–341
Ellis S, Kalinowski DS, Leotta L, Huang ML, Jelfs P, Sintchenko V, Richardson DR, Triccas JA (2014) Potent antimycobacterial activity of the pyridoxal isonicotinoyl hydrazone analog 2-pyridylcarboxaldehyde isonicotinoyl hydrazone: a lipophilic transport vehicle for isonicotinic acid hydrazide. Mol Pharm 85:269–278
Farrugia LJ (1997) ORTEP-3 for windows—a version of ORTEP-III with a graphical user interface (GUI). J Appl Cryst 30:565
Ferraz KSO, da Silva JG, Costa FM, Mendes BM, Rodrigues BL, dos Santos RG, Beraldo H (2013) N(4)-Tolyl-2-acetylpyridine thiosemicarbazones and their platinum(II, IV) and gold(III) complexes: cytotoxicity against human glioma cells and studies on the mode of action. Biometals 26:677–691
Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH (1998) Rapid, lowtechnology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar blue assay. J Clin Microbiol 36:362–366
Geary WJ (1971) The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord Chem Rev 7:81–122
Gegiou D, Lambi E, Hadjoudis E (1996) Solvatochromism in N-(2-Hydroxybenzylidene)aniline, N-(2-Hydroxybenzylidene)benzylamine, and N-(2-Hydroxybenzylidene)-2-phenylethylamine. J Phys Chem 100(45):17762–17765
Glushkov RG, Modnikova GA, L’vov IA, Krylova LY, Pushkina TV, Gus’kova TA, Solov’eva NP (2004) Synthesis and antituberculosis activity in vitro of amidine and hydrazidine analogs of pyrazinamide and isoniazid. Pharm Chem J 38:16–19
Hearn MJ, Cynamon MH, Chen MF, Coppins R, Davis J, Joo-On Kang H, Noble A, Tu-Sekine B, Terrot MS, Trombino D, Thai M, Webster ER, Wilson R (2009) Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur J Med Chem 44:4169–4178
Hoagland DT, Liu J, Lee RB, Lee RE (2016) New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv Drug Del Rev 102:55–72
Judge V, Narasimhan B, Ahuja M (2012) Isoniazid: the magic molecule. Med Chem Res 21:3940–3957
Kontos F, Maniati M, Costopoulos C, Gitti Z, Nicolaou S, Petinaki E, Anagnostou S, Tselentis I, Maniatis AN (2004) Evaluation of the fully automated Bactec MGIT 960 system for the susceptibility testing of Mycobacterium tuberculosis to first-line drugs: a multicenter study. J Microbiol Methods 56(2):291–294
Kumar HSN, Parumasivam T, Jumaat F, Ibrahim P, Asmawi MZ, Sadikun A (2014) Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med Chem Res 23(1):269–279
Lessa JA, Guerra JC, Miranda LF, Romeiro CFD, Da Silva JG, Mendes IC, Speziali NL, Souza-Fagundes EM, Beraldo H (2011) Gold(I) complexes with thiosemicarbazones: cytotoxicity against human tumor cell lines and inhibition of thioredoxin reductase activity. J Inorg Biochem 105:1729–1739
Lessa JA, Ferraz KSO, Guerra JC, de Miranda LF, Romeiro CFD, Souza-Fagundes EM, Barbeira PJS, Beraldo H (2012) Spectroscopic and electrochemical characterization of gold(I) and gold(III) complexes with glyoxaldehyde bis(thiosemicarbazones): cytotoxicity against human tumor cell lines and inhibition of thioredoxin reductase activity. Biometals 25:587–598
Lessa JA, Soares MA, dos Santos RG, Mendes IC, Salum LB, Daghestani HN, Andricopulo AD, Day BW, Vogt A, Beraldo H (2013) Gallium(III) complexes with 2-acetylpyridine-derived thiosemicarbazones: antimicrobial and cytotoxic effects and investigation on the interactions with tubulin. Biometals 26:151–165
Matei L, Bleotu C, Baciu I, Draghici C, Ionita P, Paun A, Chifiriuc MC, Sbarcea A, Zarafu I (2013) Synthesis and bioevaluation of some new isoniazid derivatives. Bioorg Med Chem 21:5355–5361
Mishra AP, Sharma N (2009) Synthesis, characterization, X-ray and thermal studies of some schiff base metal complexes. J Ind Council Chem 26(2):125–129
Mondal S, Naskara S, Deya AK, Sinnb E, Eribalb C, Herronc SR, Chattopadhyaya K (2013) Mononuclear and binuclear Cu(II) complexes of some tridentate aroyl hydrazones. X-ray crystal structures of a mononuclear and a binuclear complex. Inorg Chim Acta 398:98–105
Mosman T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Oludina YN, Voloshina AD, Kulik MV, Zobov VV, Bukharov SV, Tagasheva RG, Nugumanova GN, Burilov AR, Kravchenko MA, Skornyakov SN, Rusinov GL (2014) Synthesis, toxicity, and antituberculosis activity of isoniazid derivatives containing sterically hindered phenols. Pharm Chem J 48:5–7
Parrilha GL, Ferraz KSO, Lessa JA, de Oliveira KN, Ramos JP, Souza-Fagundes EM, Ott I, Beraldo H (2014) Metal complexes with 2-acetylpyridine-N(4)-orthochlorophenylthiosemicarbazone: cytotoxicity and effect on the enzymatic activity of thioredoxin reductase and glutathione reductase. Eur J Med Chem 84:537–544
Parumasivam T, Kumar HSN, Ibrahim P, Sadikun A, Mohamad S (2013) Anti-tuberculosis activity of lipophilic isoniazid derivatives and their interactions with first-line anti-tuberculosis drugs. J Pharm Res 7:313–317
Prozorov AA, Zaichikova MV, Danilenko VN (2012) Mycobacterium tuberculosis mutants with multidrug resistance: history of origin, genetic and molecular mechanisms of resistance, and emerging challenges. Rus J Gen 48(1):1–14
Raman N, Raja JD, Sakthivel A (2008) Design, synthesis, spectroscopic characterization, biological screening, and DNA nuclease activity of transition metal complexes derived from a tridentate Schiff base. Russ J Coord Chem 34:400–406
Ramani AV, Monika A, Indira VL, Karyavardhi G, Venkatesh J, Jeankumar VU, Manjashetty TH, Yogeeswari P, Sriram D (2012) Synthesis of highly potent novel anti-tubercular isoniazid analogues with preliminary pharmacokinetic evaluation. Bioorg Med Chem Lett 22:2764–2767
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr 64:112–122
Sorrell TN (1989) Synthetic models for binuclear copper proteins. Tetrahedron 54:10–12
Sotgiu G, Ferrara G, Matteelli A et al (2009) Epidemiology and clinical management of XDRTB: a systematic review by TBNET. Eur Respir J 33(4):871–881
Tabbì G, Giuffrida A, Bonomo RP (2013) Determination of formal redox potentials in aqueous solution of copper(II) complexes with ligands having nitrogen and oxygen donor atoms and comparison with their EPR and UV–Vis spectral features. J Inorg Biochem 128:137–145
Tortoli E, Benedetti M, Fontanelle A, Simonetti MT (2002) Evaluation of automated BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to four major antituberculous drugs: comparison with the radiometric bactec 460 tb method and the agar plate method of proportion. J Clin Microbiol 40(2):607–610
WHO (2014) Global tuberculosis Report WHO. Geneva, Switzerland: World Health Organization.http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf. Accessed 01 Aug 2016
Wong EB, Cohen KA, Bishai WR (2013) Rising to the challenge: new therapies for tuberculosis. Trends Microbiol 21(9):493–501
Zianna A, Psomas G, Hatzidimitriou A, Lalia-Kantouri M (2016) Ni(II) complexes with 2,2-dipyridylamine and salicylaldehydes: synthesis, crystal structure and interaction with calf-thymus DNA and albumins. J Inorg Biochem. doi:10.1016/j.jinorgbio
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). The authors express sincere thanks to the LDRX-UFF for the X-ray facilities and measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10534_2016_9968_MOESM1_ESM.pdf
Supplementary material 1 (PDF 890 kb). Supplementary information (infrared and UV–vis spectra used for the identification of compounds, as well as X-ray crystal data of [Cu(HAPIH)Cl]Cl·H2O (6a) and [Cu(HPzAmIH)Cl2]·H2O (8a)) is available free of charge at http://link.springer.com as PDF file. CCDC 1497179 and CCDC 1497180 contain supplementary crystallographic data for 6a and 8a, respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223- 336-033; or e-mail: deposit@ccdc.cam.ac.uk
Rights and permissions
About this article
Cite this article
Firmino, G.S.S., de Souza, M.V.N., Pessoa, C. et al. Synthesis and evaluation of copper(II) complexes with isoniazid-derived hydrazones as anticancer and antitubercular agents. Biometals 29, 953–963 (2016). https://doi.org/10.1007/s10534-016-9968-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-016-9968-7