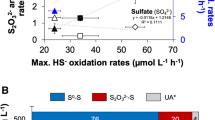
Abstract
Ferric iron in particulate iron (oxyhydr–) oxides and quinone moieties in dissolved organic matter (DOM) are well-established terminal electron acceptors (TEA) in heterotrophic anaerobic microbial respiration. The importance of particulate organic matter (POM) as TEA is, however, much less studied and understood despite the fact that POM is more abundant than DOM in many soils and sediments. Here, we studied the microbial reduction of POM and Fe(III)-containing phases in freshwater sediments. We present an electrochemical approach that can be used to assess the combined contributions of POM and Fe(III) to the TEA pools of soils and sediments. Following oxidation and drying of sediments from two carbonate–buffered freshwater lakes in air, wetting re–initiated anaerobic microbial respiration in the sediment samples as evidenced from electron transfer to solid–phase electron acceptors over three weeks of anoxic incubations. The microbial reduction of POM and mineral-associated ferric iron was directly quantified by mediated electrochemical analysis. We estimate that the POM from the analyzed sediments provided a capacity to accept or donate electrons of about 650 µmol e− (g organic carbon)−1. Our work substantiates earlier studies that suggested that the reduction of redox–active moieties in POM is an important contributor to anaerobic microbial respiration and might be responsible for the competitive suppression of methanogenesis in organic matter rich wetland soils. Our results further indicate that microbial reduction of POM must be accounted for to close respiration balances in (temporary) anoxic freshwater systems and peatlands.




Similar content being viewed by others
References
Aeschbacher M, Sander M, Schwarzenbach RP (2010) Novel electrochemical approach to assess the redox properties of humic substances. Environ Sci Technol 44(1):87–93
Aeschbacher M, Vergari D, Schwarzenbach RP, Sander M (2011) Electrochemical analysis of proton and electron transfer equilibria of the reducible moieties in humic acids. Environ Sci Technol 45:8385–8394
Aeschbacher M, Graf C, Schwarzenbach RP, Sander M (2012) Antioxidant properties of humic substances. Environ Sci Technol 46(9):4916–4925
Bauer M, Heitmann T, Macalady DL, Blodau C (2007) Electron transfer capacities and reaction kinetics of peat dissolved organic matter. Environ Sci Technol 41(1):139–145
Bethke CM, Sanford RA, Kirk MF, Jin Q, Flynn TM (2011) The thermodynamic ladder in geomicrobiology. Am J Sci 311(3):183–210
Blodau C, Deppe M (2012) Humic acid addition lowers methane release in peats of the MerBleue bog, Canada. Soil Biol Biochem 52:96–98
Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang Q (2013) Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Change Biol 19(5):1325–1346. doi:10.1111/gcb.12131
Cervantes FJ, van der Velde S, Lettinga G, Field JA (2000) Competition between methanogenesis and quinone respiration for ecologically important substrates in anaerobic consortia. FEMS Microbiol Ecol 34(2):161–171. doi:10.1111/j.1574-6941.2000.tb00766.x
Dowrick DJ, Freeman C, Lock MA, Reynolds B (2006) Sulphate reduction and the suppression of peatland methane emissions following summer drought. Geoderma 132(3–4):384–390. doi:10.1016/j.geoderma.2005.06.003
Fenner N, Freeman C (2011) Drought-induced carbon loss in peatlands. Nat Geosci 4(12):895–900
Fierer N, Schimel JP (2002) Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34(6):777–787
Gauci V, Matthews E, Dise N, Walter B, Koch D, Granberg G, Vile M (2004) Sulfur pollution suppression of the wetland methane source in the 20th and 21st centuries. Proc Natl Acad Sci USA 101(34):12583–12587. doi:10.1073/pnas.0404412101
Gorski CA, Aeschbacher M, Soltermann D, Voegelin A, Baeyens B, Marques Fernandes M, Hofstetter TB, Sander M (2012a) Redox properties of structural Fe in clay minerals: 1. Electrochemical quantification of electron donating and accepting capacities of smectites. Environ Sci Technol 46(17):9360–9368
Gorski CA, Klüpfel L, Voegelin A, Sander M, Hofstetter TB (2012b) Redox properties of structural Fe in clay minerals. 2. Electrochemical and spectroscopic characterization of electron transfer irreversibility in ferruginous smectite, SWa-1. Environ Sci Technol 46(17):9369–9377. doi:10.1021/es302014u
Gorski CA, Klüpfel LE, Voegelin A, Sander M, Hofstetter TB (2013) Redox properties of structural Fe in clay minerals: 3. Relationships between smectite redox and structural properties. Environ Sci Technol 47(23):13477–13485
Heitmann T, Goldhammer T, Beer J, Blodau C (2007) Electron transfer of dissolved organic matter and its potential significance for anaerobic respiration in a northern bog. Glob Change Biol 13(8):1771–1785. doi:10.1111/j.1365-2486.2007.01382.x
Hupfer M, Lewandowski J (2005) Retention and early diagenetic transformation of phosphorus in Lake Arendsee (Germany); consequences for management strategies. Arch Hydrobiol 164(2):143–167. doi:10.1127/0003-9136/2005/0164-0143
Kappler A, Benz M, Schink B, Brune A (2004) Electron shuttling via humic acids in microbial iron(III) reduction in a freshwater sediment. FEMS Microbiol Ecol 47(1):85–92. doi:10.1016/s0168-6496(03)00245-9
Keller JK, Bridgham SD (2007) Pathways of anaerobic carbon cycling across an ombrotrophic-minerotrophicpeatland gradient. Limnol Oceanogr 52(1):96–107
Keller JK, Takagi KK (2013) Solid-phase organic matter reduction regulates anaerobic decomposition in bog soil. Ecosphere 4(5):art54. doi:10.1890/es12-00382.1
Kjaergaard C, Heiberg L, Jensen HS, Hansen HCB (2012) Phosphorus mobilization in rewetted peat and sand at variable flow rate and redox regimes. Geoderma 173–174:311–321. doi:10.1016/j.geoderma.2011.12.029
Kluepfel L, Keiluweit M, Kleber M, Sander M (2014a) Redox properties of plant biomass-derived black carbon (biochar). Environ Sci Technol 48(10):5601–5611. doi:10.1021/es500906d
Kluepfel L, Piepenbrock A, Kappler A, Sander M (2014b) Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat Geosci 7(3):195–200. doi:10.1038/ngeo2084
Knösche R (2006) Organic sediment nutrient concentrations and their relationship with the hydrological connectivity of floodplain waters (River Havel, NE Germany). Hydrobiologia 560(1):63–76. doi:10.1007/s10750-005-0983-x
Lalonde K, Mucci A, Ouellet A, Gélinas Y (2012) Preservation of organic matter in sediments promoted by iron. Nature 483(7388):198–200
LaRowe DE, Van Cappellen P (2011) Degradation of natural organic matter: a thermodynamic analysis. Geochim Cosmochim Acta 75(8):2030–2042. doi:10.1016/j.gca.2011.01.020
Lewandowski J, Laskov C, Hupfer M (2007) The relationship between Chironomusplumosus burrows and the spatial distribution of pore-water phosphate, iron and ammonium in lake sediments. Freshw Biol 52(2):331–343. doi:10.1111/j.1365-2427.2006.01702.x
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJ, Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382(6590):445–448
Martinez C, Alvarez L, Celis L, Cervantes F (2013) Humus-reducing microorganisms and their valuable contribution in environmental processes. Appl Microbiol Biotechnol 97(24):10293–10308. doi:10.1007/s00253-013-5350-7
Megonigal JP, Hines M, Visscher P (2003) 8.08—Anaerobic metabolism: linkages to trace gases and Aerobic processes. In: Holland HD, Turekian KK (eds) Treatise on geochemistry, vol 8. Pergamon, Oxford, pp 317–424. doi:10.1016/B0-08-043751-6/08132-9
Piccolo A (2001) The supramolecular structure of humic substances. Soil Sci 166(11):810–832
Postma D, Jakobsen R (1996) Redox zonation: equilibrium constraints on the Fe(III)/SO4-reduction interface. Geochim Cosmochim Acta 60(17):3169–3175. doi:10.1016/0016-7037(96)00156-1
Qiu S, McComb A (1995) Planktonic and microbial contributions to phosphorus release from fresh and air-dried sediments. Aust J Mar Freshw Res 46(7):1039–1045
Ratasuk N, Nanny MA (2007) Characterization and quantification of reversible redox sites in humic substances. Environ Sci Technol 41(22):7844–7850
Ratering S, Schnell S (2000) Localization of iron-reducing activity in paddy soilby profile studies. Biogeochemistry 48(3):341–365. doi:10.1023/a:1006252315427
Roden EE, Wetzel RG (1996) Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr 41(8):1733–1748
Roden EE, Kappler A, Bauer I, Jiang J, Paul A, Stoesser R, Konishi H, Xu H (2010) Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nat Geosci 3(6):417–421. doi:10.1038/ngeo870
Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE, Lovley DR (1998) Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol 32(19):2984–2989
Shotyk W (1988) Review of the inorganic geochemistry of peats and peatland waters. Earth-Sci Rev 25(2):95–176. doi:10.1016/0012-8252(88)90067-0
Sposito G (2011) Electron shuttling by natural organic matter: twenty years after. In: Aquatic redox chemistry, vol 1071. ACS Symposium Series, vol 1071. American Chemical Society, pp 113–127. doi:10.1021/bk-2011-1071.ch006
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions, vol 2. John Wiley & Sons, New York
Struyk Z, Sposito G (2001) Redox properties of standard humic acids. Geoderma 102(3–4):329–346. doi:10.1016/S0016-7061(01)00040-4
Stumm W, Morgan JJ (1996) Aquatic Chemistry, Chemical equilibria and rates in natural water. Wiley-Interscience, New York
Sutton R, Sposito G (2005) Molecular structure in soil humic substances: the new view. Environ Sci Technol 39(23):9009–9015
Tamura H, Goto K, Yotsuyanagi T, Nagayama M (1974) Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta 21(4):314–318. doi:10.1016/0039-9140(74)80012-3
Tranvik LJ, Downing JA, Cotner JB, Loiselle SA, Striegl RG, Ballatore TJ, Dillon P, Finlay K, Fortino K, Knoll LB (2009) Lakes and reservoirs as regulators of carbon cycling and climate. Limnol Oceanogr 54(6):2298–2314
Uchimiya M, Stone A (2010) Reduction of substituted p-Benzoquinones by Fe(II) near neutral pH. Aquat Geochem 16(1):173–188. doi:10.1007/s10498-009-9077-0
Wilson JS, Baldwin DS (2008) Exploring the ‘Birch effect’ in reservoir sediments: influence of inundation history on aerobic nutrient release. Chem Ecol 24(6):379–386
Yao H, Conrad R, Wassmann R, Neue H (1999) Effect of soil characteristics on sequential reduction and methane production in sixteen rice paddy soils from China, the Philippines, and Italy. Biogeochemistry 47(3):269–295
Zak D, Kleeberg A, Hupfer M (2006) Sulphate-mediated phosphorus mobilization in riverine sediments at increasing sulphate concentration, River Spree, NE Germany. Biogeochemistry 80(2):109–119
Zak D, Rossoll T, Exner H-J, Wagner C, Gelbrecht J (2009) Mitigation of sulfate pollution by rewetting of fens—a conflict with restoring their phosphorus sink function? Wetlands 29(4):1093–1103
Acknowledgments
We thank Hans-Juergen Exner for analytical assistance, Laura Klüpfel for introducing Maximilian Lau to mediated electrochemical analysis and Dominik Zak for helpful discussions. We also thank three anonymous reviewers for helpful comments and suggestions. Funding was provided by the Leibniz Association (SAW-2012-IGB-4167).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Robert Cook.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lau, M.P., Sander, M., Gelbrecht, J. et al. Solid phases as important electron acceptors in freshwater organic sediments. Biogeochemistry 123, 49–61 (2015). https://doi.org/10.1007/s10533-014-0052-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-0052-5