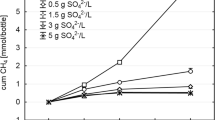
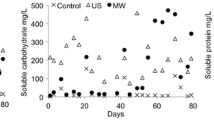
Abstract
Performance stability is a key issue when managing anaerobic digesters. However it can be affected by external disturbances caused by micropollutants. In this study the influence of phenol on the methanization of cellulose was evaluated through batch toxicity assays. Special attention was given to the dynamics of microbial communities by means of automated ribosomal intergenic spacer analysis. We observed that, as phenol concentrations increased, the different steps of anaerobic cellulose digestion were unevenly and progressively affected, methanogenesis being the most sensitive: specific methanogenic activity was half-inhibited at 1.40 g/L of phenol, whereas hydrolysis of cellulose and its fermentation to VFA were observed at up to 2.00 g/L. Depending on the level of phenol, microbial communities resisted either through physiological or structural adaptation. Thus, performances at 0.50 g/L were maintained in spite of the microbial community’s shift. However, the communities’ ability to adapt was limited and performances decreased drastically beyond 2.00 g/L of phenol.





Similar content being viewed by others
References
Angelidaki I, Mogensen AS, Ahring BK (2000) Degradation of organic contaminants found in organic waste. Biodegradation 11:377–383
Barnes SP, Keller J (2003) Cellulosic waste degradation by rumen-enhanced anaerobic digestion. Water Sci Technol 48:155–162
Bayer EA, Lamed R, Himmel ME (2007) The potential of cellulases and cellulosomes for cellulosic waste management. Curr Opin Biotechnol 18:237–245
Blum DJW, Hergenroeder R, Parkin GF, Speece RE (1986) Anaerobic treatment of coal conversion wastewater constituents: Biodegradability and toxicity. J Water Pollut Control Fed 58:122–131
Cardinale M et al (2004) Comparison of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Appl Environ Microbiol 70:6147–6156
Chapleur O, Bize A, Serain T, Mazeas L, Bouchez T (2014) Co-inoculating ruminal content neither provides active hydrolytic microbes nor improves methanization of (1)(3)C-cellulose in batch digesters. FEMS Microbiol Ecol 87:616–629. doi:10.1111/1574-6941.12249
Chapleur O, Mazeas L, Godon JJ, Bouchez T (2015) Asymmetrical response of anaerobic digestion microbiota to temperature changes. Appl Microbiol Biotechnol. doi:10.1007/s00253-015-7046-7
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99:4044–4064
Fang HHP, Chan O-C (1997) Toxicity of phenol towards anaerobic biogranules. Water Res 31:2229–2242
Fang HHP, Chen T, Chan OC (1995) Toxic effects of phenolic pollutants on anaerobic benzoate-degrading granules. Biotechnol Lett 17:117–120. doi:10.1007/BF00134207
Fechner LC, Gourlay-Francé C, Uher E, Tusseau-Vuillemin MH (2010a) Adapting an enzymatic toxicity test to allow comparative evaluation of natural freshwater biofilms’ tolerance to metals. Ecotoxicology 19:1302–1311. doi:10.1007/s10646-010-0517-9
Fechner LC, Vincent-Hubert F, Gaubert P, Bouchez T, Gourlay-Francé C, Tusseau-Vuillemin MH (2010b) Combined eukaryotic and bacterial community fingerprinting of natural freshwater biofilms using automated ribosomal intergenic spacer analysis. FEMS Microbiol Ecol 74:542–553
Fedorak PM, Hrudey SE (1984) The effects of phenol and some alkyl phenolics on batch anaerobic methanogenesis. Water Res 18:361–367
Fisher MM, Triplett EW (1999) Automated approach for ribosomal intergenic spacer analysis of microbial diversity and its application to freshwater bacterial communities. Appl Environ Microbiol 65:4630–4636
García-Martínez J, Rodríguez-Valera F (2000) Microdiversity of uncultured marine prokaryotes: the SAR11 cluster and the marine Archaea of group I. Mol Ecol 9:935–948. doi:10.1046/j.1365-294X.2000.00953.x
Hernandez JE, Edyvean RGJ (2008) Inhibition of biogas production and biodegradability by substituted phenolic compounds in anaerobic sludge. J Hazard Mater 160:20–28. doi:10.1016/j.jhazmat.2008.02.075
Hoyos-Hernandez C, Hoffmann M, Guenne A, Mazeas L (2014) Elucidation of the thermophilic phenol biodegradation pathway via benzoate during the anaerobic digestion of municipal solid waste. Chemosphere 97:115–119
Khardenavis AA, Kapley A, Purohit HJ (2008) Phenol-mediated improved performance of active biomass for treatment of distillery wastewater. Int Biodeterior Biodegrad 62:38–45
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26
Levén L, Nyberg K, Korkea-aho L, Schnürer A (2006) Phenols in anaerobic digestion processes and inhibition of ammonia oxidising bacteria (AOB) in soil. Sci Total Environ 364:229–238
Loy A et al (2002) Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. Appl Environ Microbiol 68:5064–5081. doi:10.1128/aem.68.10.5064-5081.2002
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Michelland RJ, Dejean S, Combes S, Fortun-Lamothe L, Cauquil L (2009) StatFingerprints: a friendly graphical interface program for processing and analysis of microbial fingerprint profiles. Mol Ecol Resour 9:1359–1363
Monlau F, Sambusiti C, Barakat A, Quéméneur M, Trably E, Steyer JP, Carrère H (2014) Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? Compr Rev Biotechnol Adv 32:934–951. doi:10.1016/j.biotechadv.2014.04.007
Olguin-Lora P, Puig-Grajales L, Razo-Flores E (2003) Inhibition of the acetoclastic methanogenic activity by phenol and alkyl phenols. Environ Technol 24:999–1006. doi:10.1080/09593330309385638
Prosser JI (2010) Replicate or lie. Environ Microbiol 12:1806–1810. doi:10.1111/j.1462-2920.2010.02201.x
Rosenkranz F et al (2013) Relationship between phenol degradation efficiency and microbial community structure in an anaerobic SBR. Water Res 47:6739–6749. doi:10.1016/j.watres.2013.09.004
Sierra-Alvarez R, Lettinga G (1991) The effect of aromatic structure on the inhibition of acetoclastic methanogenesis in granular sludge. Appl Microbiol Biotechnol 34:544–550
Thioulouse J, Chessel D, Dole´dec S, Olivier J-M (1997) ADE-4: a multivariate analysis and graphical display software. Stat Comput 7:75–83. doi:10.1023/A:1018513530268
van Dorst J et al (2014) Community fingerprinting in a sequencing world. FEMS Microbiol Ecol 89:316–330. doi:10.1111/1574-6941.12308
Veeresh GS, Kumar P, Mehrotra I (2005) Treatment of phenol and cresols in upflow anaerobic sludge blanket (UASB) process: a review. Water Res 39:154–170
Wang YT, Suidan MT, Pfeffer TJ, Najam I (1989) The effect of concentration of phenols on their batch methanogenesis. Biotechnol Bioeng 33:1353–1357
Wang YT, Gabbard HD, Pai PC (1991) Inhibition of acetate methanogenesis by phenols. J Environ Eng 117:487–500
Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85:849–860. doi:10.1007/s00253-009-2246-7
Wu JJ, Masten SJ (2002) Oxidation kinetics of phenolic and indolic compounds by ozone: applications to synthetic and real swine manure slurry. Water Res 36:1513–1526. doi:10.1016/S0043-1354(01)00352-9
Acknowledgments
We are grateful to Julien Guieu for the English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10532_2015_9751_MOESM1_ESM.tiff
Fig. S1 Methane production over time. Methane production (mg of C) over time (number of days) for the different groups of triplicate bioreactors (mean values, error bars represent standard deviation within triplicates) (TIFF 46406 kb)
10532_2015_9751_MOESM2_ESM.tiff
Fig. S2 Carbon dioxide production over time. Carbon dioxide production (mg of C) over time (number of days) for the different groups of triplicate bioreactors (mean values, error bars represent standard deviation within triplicates) (TIFF 46406 kb)
10532_2015_9751_MOESM3_ESM.tiff
Fig. S3 pH over time. pH over time (number of days) for the different groups of triplicate bioreactors (mean values, error bars represent standard deviation within triplicates) (TIFF 46406 kb)
10532_2015_9751_MOESM4_ESM.tiff
Fig. S4 Concentration of dissolved organic carbon over time. Dissolved organic carbon (mg of C per L) over time (number of days) for the different groups of triplicate bioreactors (mean values, error bars represent standard deviation within triplicates) (TIFF 46406 kb)
10532_2015_9751_MOESM5_ESM.tif
Fig. S5 Principal Component Analysis (PCA) of respectively archaeal and bacterial diversity profiles generated by ARISA. The color scale represents the date of sampling (respectively A and B) or the concentration of phenol measured at the date of sampling (respectively C and D). For Archaea, the first and second axes of the PCA provided the clearest separation of ARISA profiles, with resp. 36.9 % and 21.5 % of the total variance, while for Bacteria, the first and third axes provided the clearest separation, with resp. 40.8 % and 13.0 % of the total variance (TIFF 11748 kb)
10532_2015_9751_MOESM6_ESM.tif
Fig. S6 Principal Component Analysis (PCA) of bacterial diversity profiles generated by ARISA. The color scale represents the initial phenol concentration in the bioreactors. The first and second axes of the PCA provided the clearest separation of ARISA profiles, with resp. 32.7 % and 26.2 % of the total variance (TIFF 1953 kb)
Rights and permissions
About this article
Cite this article
Chapleur, O., Madigou, C., Civade, R. et al. Increasing concentrations of phenol progressively affect anaerobic digestion of cellulose and associated microbial communities. Biodegradation 27, 15–27 (2016). https://doi.org/10.1007/s10532-015-9751-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-015-9751-4