Abstract
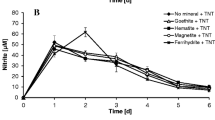
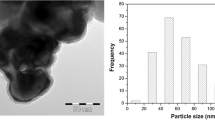
This study investigated the impact of ferrihydrite on the pathway and rate of 2,4,6-trinitrotoluene (TNT) transformation by Yarrowia lipolytica AN-L15. The presence of ferrihydrite in the culture medium decreased the rate of TNT biotransformation but resulted in the accumulation of the same TNT metabolites as in the absence of ferrihydrite, albeit at slightly different concentrations. Transformation products observed included aromatic ring reduction products, such as hydride-Meisenheimer complexes, and nitro group reduction products, such as hydroxylamino- and amino-dinitrotoluenes. Independently of the presence of ferrihydrite the subsequent degradation of the hydride complex(es) resulted in the release of nitrite followed by its conversion to nitrate and nitric oxide at the low pH values observed during yeast cultivation. Nitric oxide generation was ascertained by electron spin resonance spectroscopy. In addition, increased Fe3+-reduction was observed in the presence of TNT and Y. lipolytica. This study demonstrates that in the presence of yeast cells, TNT-hydride complexes were formed at approximately the same level as in the presence of ferrihydrite, opening up the possibility of aromatic ring cleavage, instead of promoting the production of potentially toxic nitro group reduction products in the presence of iron minerals.





Similar content being viewed by others
References
Adamia G, Ghoghoberidze M, Graves D, Khatisashvili G, Kvesitadze G, Lomidze E, Ugrekhelidze D, Zaalishvili G (2006) Absorption, distribution, and transformation of TNT in higher plants. Ecotoxicol Environ Saf 64:136–145
Agrawal A, Tratnyek PG (1996) Reduction of nitro aromatic compounds by zero-valent iron metal. Environ Sci Technol 30:153–160
Aguiar A, de Souza-Cruz PB, Ferraz A (2006) Oxalic acid, Fe3+-reduction activity and oxidative enzymes detected in culture extracts recovered from Pinus taeda wood chips biotreated by Ceriporiopsis subvermispora. Enzym Microb Technol 38:873–878
Boparai HK, Comfort SD, Shea PJ, Szecsody JE (2008) Remediating explosive-contaminated groundwater by in situ redox manipulation (ISRM) of aquifer sediments. Chemosphere 71:933–941
Boparai HK, Comfort SD, Satapanajaru T, Szecsody JE, Grossl PR, Shea PJ (2010) Abiotic transformation of high explosives by freshly precipitated iron minerals in aqueous FeII solutions. Chemosphere 79:865–872
Borch T, Gerlach R (2004) Use of reversed-phase high-performance liquid chromatography–diode array detection for complete separation of 2,4,6-trinitrotoluene metabolites and EPA Method 8330 explosives: influence of temperature and an ion-pair reagent. J Chromatogr A 1022:83–94
Borch T, Inskeep WP, Harwood JA, Gerlach R (2005) Impact of ferrihydrite and anthraquinone-2,6-disulfonate on the reductive transformation of 2,4,6-trinitrotoluene by a gram-positive fermenting bacterium. Environ Sci Technol 39:7126–7133
Cai Q, Zhang W, Yang Z (2001) Stability of nitrite in wastewater and its determination by ion chromatography. Anal Sci 17:917–920
Coby AJ, Picardal FW (2005) Inhibition of NO3 − and NO2 − reduction by microbial Fe(III) reduction: evidence of a reaction between NO2 − and cell surface bound Fe2+. Appl Environ Microbiol 71:5267–5274
Cudennec Y, Lecerf A (2006) The transformation of ferrihydrite into goethite or hematite, revisited. J Solid State Chem 179:716–722
Eilers A, Rüngeling E, Stündl UM, Gottschalk G (1999) Metabolism of 2,4,6-trinitrotoluene by the white-rot fungus Bjerkandera adusta DSM 3375 depends on cytochrome P-450. Appl Microbiol Biotechnol 53:75–80
Esteve-Núñez A, Caballero A, Ramos JL (2001) Biological degradation of 2,4,6-trinitrotoluene. Microbiol Mol Biol Rev 65:335–352
Eyers L, Stenuit B, Agathos SN (2008) Denitration of 2,4,6-trinitrotoluene by Pseudomonas aeruginosa ESA-5 in the presence of ferrihydrite. Appl Microbiol Biotechnol 79:489–497
Fiorella PD, Spain JC (1997) Transformation of 2,4,6-trinitrotoluene by Pseudomonas pseudoalcaligenes JS52. Appl Environ Microbiol 63:2007–2015
French CE, Nicklin S, Bruce NC (1998) Aerobic degradation of 2,4,6-trinitrotoluene by Enterobacter cloacae PB2 and by pentaerythritol tetranitrate reductase. Appl Environ Microbiol 64:2864–2868
Fujii H, Berliner LJ (1999) Ex vivo EPR detection of nitric oxide in brain tissue. Magn Reson Med 42:599–602
Hannink NK, Rosser SJ, French CE, Bruce NC (2003) Uptake and metabolism of TNT and GTN by plants expressing bacterial pentaerythritol tetranitrate reductase. Water Air Soil Pollut 3:251–258
Harter DR (1985) The use and importance of nitroaromatic chemicals in the chemical industry. In: Rickert DE (ed) Toxicity of nitroaromatic compounds. Hemisphere Publishing Corporation, Washington, pp 1–14
Hawari J, Halasz A, Beaudet S, Paquet L, Ampleman G, Thiboutot S (1999) Biotransformation of 2,4,6-trinitrotoluene with Phanerochaete chrysosporium in agitated cultures at pH 4.5. Appl Environ Microbiol 65:2977–2986
Heiss G, Knackmuss HJ (2002) Bioelimination of trinitroaromatic compounds: immobilization versus mineralization. Curr Opin Microbiol 5:282–287
Hofstetter TB, Heijman CG, Haderlein SB, Holliger C, Schwarzenbach RP (1999) Complete reduction of TNT and other (poly)nitroaromatic compounds under iron-reducing subsurface conditions. Environ Sci Technol 33:1479–1487
Hofstetter TB, Neumann A, Schwarzenbac RP (2006) Reduction of nitroaromatic compounds by Fe(II) species associated with iron-rich smectites. Environ Sci Technol 40:235–242
Huang S, Lindahl PA, Wang C, Bennett GN, Rudolph FB, Hughes JB (2000) 2,4,6-Trinitrotoluene reduction by carbon monoxide dehydrogenase from Clostridium thermoaceticum. Appl Environ Microbiol 66:1474–1478
Hundal LS, Singh J, Bier EL, Shea PJ, Comfort SD, Powers WL (1997) Removal of TNT and RDX from water and soil using iron metal. Environ Pollut 97:55–64
Leung KH, Yao M, Stearns R, Chiu S-HL (1995) Mechanism of bioactivation and covalent binding of 2,4,6-trinitrotoluene. Chem Biol Interact 97:37–51
Lovley DR, Phillips EJP (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 53:1536–1540
Michels J, Gottschalk G (1994) Inhibition of lignin peroxidase of Phanerochaete chrysosporium by hydroxylamino-dinitrotoluene, an early intermediate in the degradation of 2,4,6-trinitrotoluene. Appl Environ Microbiol 60:187–194
Morgan B, Lahav O (2007) The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution—basic principles and a simple heuristic description. Chemosphere 68:2080–2084
Nefso EK, Burns SE, McGrath CJ (2005) Degradation kinetics of TNT in the presence of six mineral surfaces and ferrous iron. J Hazard Mater 123:79–88
Nepovim A, Hebner A, Soudek P, Gerth A, Thomas H, Smrcek S, Vanek T (2005) Degradation of 2,4,6-trinitrotoluene by selected helophytes. Chemosphere 60:1454–1461
Oh SY, Cha DK, Kim BJ, Chiu PC (2002) Effect of adsorption to elemental iron on the transformation of 2,4,6-trinitrotoluene and hexahydro-1,3,5-trinitro-1,3,5-triazine in solution. Environ Toxicol Chem 21:1384–1389
Pak JW, Knoke KL, Noguera DR, Fox BG, Chambliss GH (2000) Transformation of 2,4,6-trinitrotoluene by purified xenobiotic reductase B from Pseudomonas fluorescens I-C. Appl Environ Microbiol 66:4742–4750
Rieger PG, Knackmuss HJ (1995) Basic knowledge and perspectives on biodegradation of 2,4,6-TNT and related nitroaromatic compounds in contaminated soil. In: Spain JC (ed) Biodegradation of nitroaromatic compounds. Plenum, New York, pp 1–18
Rylott EL, Lorenz A, Bruce NC (2011) Biodegradation and biotransformation of explosives. Curr Opin Biotechnol 22:434–440
Scheibner K, Hofrichter M, Herre A, Michels J, Fritsche W (1997) Screening for fungi intensively mineralizing 2,4,6-trinitrotoluene. Appl Microbiol Biotechnol 47:452–457
Singh B, Kaur J, Singh K (2012) Microbial remediation of explosive waste. Crit Rev Microbiol 38:152–167
Spain JC (1995) Biodegradation of nitroaromatic compounds. Annu Rev Microbiol 49:523–555
Stenuit BA, Agathos SN (2010) Microbial 2,4,6-trinitrotoluene degradation: could we learn from (bio)chemistry for bioremediation and vice versa? Appl Microbiol Biotechnol 88:1043–1064
Stenuit BA, Eyers L, Fantroussi SE, Agathos SN (2005) Promising strategies for the mineralization of 2,4,6-trinitrotoluene. Rev Environ Sci Bio/Technol 4:39–60
Ueno T, Suzuki Y, Fujii S, Vanin AF, Yoshimura T (2002) In vivo nitric oxide transfer of a physiological NO carrier, dinitrosyl dithiolato iron complexes, to target complexes. Biochem Pharmacol 63:485–493
van Aken B, Hofrichter M, Scheibner K, Hatakka AI, Naveau H, Agathos SN (1999) Transformation and mineralization of 2,4,6-trinitrotoluene (TNT) by manganese peroxidase from the white-rot basidiomycete Phlebia radiate. Biodegradation 10:83–91
van Dillewijn P, Couselo JL, Corredoira E, Delgado A, Wittich RM, Ballester A, Ramos JL (2008a) Bioremediation of 2,4,6-trinitrotoluene by bacterial nitroreductase expressing transgenic aspen. Environ Sci Technol 42:7405–7410
van Dillewijn P, Wittich RM, Caballero A, Ramos JL (2008b) Type II hydride transferases from different microorganisms yield nitrite and diarylamines from polynitroaromatic compounds. Appl Environ Microbiol 74:6820–6823
Vanin AF, Liu X, Samouilov A, Stukan RA, Zweier JL (2000) Redox properties of iron-dithiocarbamates and their nitrosyl derivatives: implications for their use as traps of nitric oxide in biological systems. Biochim Biophys Acta 1474:365–377
Vorbeck C, Lenke H, Fischer P, Spain JC, Knackmuss HJ (1998) Initial reductive reactions in aerobic microbial metabolism of 2,4,6-trinitrotoluene. Appl Environ Microbiol 64:246–252
Wittich RM, Ramos JL, van Dillewijn P (2009) Microorganisms and explosives: mechanisms of nitrogen release from TNT for use as an N-source for growth. Environ Sci Technol 43:2773–2776
Ye J, Singh A, Ward OP (2004) Biodegradation of nitroaromatics and other nitrogen-containing xenobiotics. World J Microbiol Biotechnol 20:117–135
Yinon J (1990) Toxicity and metabolism of explosives. CRC Press, Boca Raton
Ziganshin AM, Gerlach R, Borch T, Naumov AV, Naumova RP (2007) Production of eight different hydride complexes and nitrite release from 2,4,6-trinitrotoluene by Yarrowia lipolytica. Appl Environ Microbiol 73:7898–7905
Ziganshin AM, Naumova RP, Pannier AJ, Gerlach R (2010a) Influence of pH on 2,4,6-trinitrotoluene degradation by Yarrowia lipolytica. Chemosphere 79:426–433
Ziganshin AM, Gerlach R, Naumenko EA, Naumova RP (2010b) Aerobic degradation of 2,4,6-trinitrotoluene by the yeast strain Geotrichum candidum AN-Z4. Microbiology 79:199–205
Acknowledgments
This work was supported by a grant from the Ministry of Education and Science of the Russian Federation (2010–2011) and partially supported by a grant “Alğarış” from the Republic of Tatarstan (Russia). Partial financial support was provided by the US Department of Defense, Army Research Office, Grant No. DAAD19-03-C-0103 and the Office of Science (BER), U.S. Department of Energy, Grant No. DE-FG02-03ER63582. The authors acknowledge funding for the establishment of the Environmental and Biofilm Mass Spectrometry Facility through the Defense University Research Instrumentation Program (DURIP) Contract No. W911NF0510255. We gratefully acknowledge Alexander Rodionov (Institute of Physics, Kazan Federal University) for ESR spectroscopy assistance.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khilyas, I.V., Ziganshin, A.M., Pannier, A.J. et al. Effect of ferrihydrite on 2,4,6-trinitrotoluene biotransformation by an aerobic yeast. Biodegradation 24, 631–644 (2013). https://doi.org/10.1007/s10532-012-9611-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-012-9611-4