Abstract
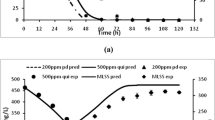
Many contaminated sites commonly have complex mixtures of polycyclic aromatic hydrocarbons (PAHs) whose individual microbial biodegradation may be altered in mixtures. Biodegradation kinetics for fluorene, naphthalene, 1,5-dimethylnaphthalene and 1-methylfluorene were evaluated in sole substrate, binary and ternary systems using Sphingomonas paucimobilis EPA505. The first order rate constants for fluorene, naphthalene, 1,5-dimethylnaphthalene, and 1-methylfluorene were comparable; yet Monod parameters were significantly different for the tested PAHs. S. paucimobilis completely degraded all the components in binary and ternary mixtures; however, the initial degradation rates of individual components decreased in the presence of competitive PAHs. Results from the mixture experiments indicate competitive interactions, demonstrated mathematically. The generated model appropriately predicted the biodegradation kinetics in mixtures using parameter estimates from the sole substrate experiments, validating the hypothesis of a common rate-determining step. Biodegradation kinetics in mixtures were affected by the affinity coefficients of the co-occurring PAHs and mixture composition. Experiments with equal concentrations of substrates demonstrated the effect of concentration on competitive inhibition. Ternary experiments with naphthalene, 1,5-dimethylnaphthalene and 1-methylfluorene revealed delayed degradation, where depletion of naphthalene and 1,5-dimethylnapthalene occurred rapidly only after the complete removal of 1-methylfluorene. The substrate interactions observed in mixtures require a multisubstrate model to account for simultaneous degradation of substrates. PAH contaminated sites are far more complex than even ternary mixtures; however these studies clearly demonstrate the effect that interactions can have on individual chemical kinetics. Consequently, predicting natural or enhanced degradation of PAHs cannot be based on single compound kinetics as this assumption would likely overestimate the rate of disappearance.




Similar content being viewed by others
References
Bauer JE, Capone DG (1988) Effects of co-occurring aromatic hydrocarbons on degradation of individual aromatic hydrocarbons in marine sediment slurries. Appl Environ Microbiol 54:1649–1655
Beckles DM, Ward CH, Hughes JB (1998) Effects of mixtures of PAHs and sediments on fluoranthene biodegradation patterns. Environ Toxicol Chem 17:1246–1251
Boldrin B, Tiehm A, Fritzche C (1993) Degradation of phenanthrene, fluorene, fluoranthene, and pyrene by a Mycobacterium sp. Appl Environ Microbiol 59:1927–1930
Bouchez M, Blanchet D, Vandecasteele J-P (1995) Degradation of polycyclic hydrocarbons by pure strains and by defined strain associations: Inhibition phenomena and cometabolism. Appl Microbiol Biotechnol 43:156–164
Bradford MM (1976) A rapid & sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cerniglia CE (1992) Biodegradation of PAHs. Biodegradation 3:351–368
Dabestani R, Ivanov IN (1999) A compilation of physical, spectroscopic and photophysical properties of PAHs. Photochem Photobiol 70:10–34
Dimitriou-Christidis P (2005) Modeling the biodegradability and physicochemical properties of PAHs. Dissertation, Texas A&M University, College Station
Ellis TG, Barbeau DS, Smets BF, Grady CPL (1996) Respirometric technique for determination of extant kinetic parameters describing biodegradation. Water Environ Res 68:917–926
Grady CPL, Smets BF, Barbeau DS (1996) Variability in kinetic parameter estimates: A review of possible causes and a proposed terminology. Wat Res 30:742–748
Guha S, Peters CA, Jaffé PR (1999) Multisubstrate biodegradation kinetics of naphthalene, phenanthrene, and pyrene mixtures. Biotechnol Bioeng 65:491–499
Guha S, Jaffé PR, Peters CA (1998) Solubilization of PAH mixtures by a nonionic surfactant. Environ Sci Technol 32:930–935
Heitkamp MA, Cerniglia CE (1988) Mineralization of PAHs by a bacterium isolated from sediment below an oil field. Appl Environ Microbiol 54:1612–1614
Kanaly RA, Harayama S (2000) Biodegradation of high-molecular-weight PAHs by bacteria. J Bacteriol 182:2059–2067
Kelley I, Cerniglia CE (1995) Degradation of a mixture of high-molecular-weight polycyclic aromatic hydrocarbons by a Mycobacterium strain PYR-1. J Soil Contam 4:77–91
Knightes CD (2000) Mechanisms governing sole-substrate and multi-substrate biodegradation kinetics of polycyclic aromatic hydrocarbons. Dissertation, Princeton University, Princeton
Knightes CD, Peters CA (2000) Aqueous phase biodegradation kinetics of 10 PAH compounds. Environ Eng Sci 20:207–217
Kovárová-Kovar K, Egli T (1998) Growth kinetics of suspended microbial cells: from single-substrate-controlled growth to mixed-substrate kinetics. Microbiol Mol Biol Rev 62:646–666
Lantz SE, Montgomery MT, Schultz WW, Pritchard PH, Spargo BJ, Mueller JG (1997) Constituents of an organic wood preservative that inhibit the fluoranthene-degrading activity of Sphingomonas paucimobilis strain EPA505. Environ Sci Technol 31:3573–3580
Leblond JD, Schultz TW, Sayler GS (2001) Observations on the preferential biodegradation of selected components of polyaromatic hydrocarbon mixtures. Chemosphere 42:333–343
Luning Prak DJ, Pritchard PH (2002) Degradation of PAHs dissolved in Tween 80 surfactant solutions by Sphingomonas paucimobilis EPA505. Can J Microbiol 48:151–158
Mackay D, Shiu YW, Ma KC (1992) Illustrated handbook of physical-chemical properties and environmental fate for organic chemicals. Lewis Publishers, Chelsea, MI
Mohan SV, Kisa T, Ohkuma T, Robert AK, Shimizu Y (2006) Bioremediation technologies for treatment of PAH-contaminated soil and strategies to enhance process efficiency. Rev Environ Sci Biotechnol 5:347–374
Molina M, Araujo R, Hodson RE (1999) Cross-induction of pyrene and phenanthrene in a Mycobacterium sp. isolated from PAHs contaminated river sediments. Can J Microbiol 45:520–529
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3:371–394
Mueller JG, Chapman PJ, Blattmann BO, Pritchard PH (1989) Action of a fluoranthene-utilizing bacterial community on PAH components of creosote. Appl Environ Microbiol 55:3085–3090
Mueller JG, Chapman PJ, Blattmann BO, Pritchard PH (1990) Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. Appl Environ Microbiol 56:1079–1086
Segel IH (1975) Enzyme kinetics. John Wiley & Sons, New York
Sherma J (1993) Handbook of chromatography. CRC Press, Inc., Boca Raton, FL
Smith LH, McCarty PL, Kitanidis PK (1998) Spreadsheet method for evaluation of biochemical reaction rate coefficients and their uncertainties by weighted nonlinear least-squares analysis of the integrated Monod equation. Appl Environ Microbiol 64:2044–2050
Stringfellow WT, Aitken MD (1995) Competitive metabolism of naphthalene, methylnaphthalenes, and fluorene by phenanthrene-degrading Pseudomonads. Appl Environ Microbiol 61:357–362
U.S. Environmental Protection Agency (1989) The superfund innovative technology programme: Technology profiles. EPA Report No. 540/5–89/01. US EPA Risk Reduction Engineering Laboratory Office of Research and Development, Cincinnati, OH. In: Wilson SC, Jones KC (1993) Bioremediation of soil contaminated with PAHs: A review. Environ. Pollution 81:229–249
Ye D, Siddiqi MA, Maccubbin AE, Kumar S, Sikka HC (1996) Degradation of PAHs by Sphingomonas paucimobilis. Environ Sci Technol 30:136–142
Acknowledgements
We thank the Superfund Basic Research Program at Texas A&M University that supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desai, A.M., Autenrieth, R.L., Dimitriou-Christidis, P. et al. Biodegradation kinetics of select polycyclic aromatic hydrocarbon (PAH) mixtures by Sphingomonas paucimobilis EPA505. Biodegradation 19, 223–233 (2008). https://doi.org/10.1007/s10532-007-9129-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-007-9129-3