Abstract
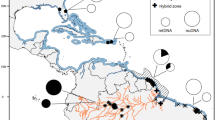
Mediterranean red-legged (Alectoris rufa) and rock (Alectoris graeca) partridge populations are affected by genetic pollution. The chukar partridge (Alectoris chukar), a species only partly native to Europe, is the most frequently introgressive taxon detected in the genome of hybrid partridges. Both theoretical (evolutionary) and practical (resources management) matters spur to get insight into the geographic origin of the A. chukar hybridizing swarm. The phenotypic A. rufa populations colonizing the easternmost part of the distribution range of this species, the islands of Elba (Italy) and Corsica (France), were investigated. The analysis of both mitochondrial (mtDNA: Cytochrome-b gene plus Control Region: 2,250 characters) and nuclear (Short Tandem Repeats, STR; Random Amplified Polymorphic DNA, RAPD) genomes of 25 wild (Elba) and 20 captive (Corsica) partridges, disclosed spread introgression of chukar origin also in these populations. All mtDNA haplotypes of Elba and Corsica partridges along with those we obtained from other A. rufa (total, n = 111: Italy, Spain, France) and A. graeca (n = 6, Italy), were compared with the mtDNA haplotypes of chukars (n = 205) sampled in 20 countries. It was found that the A. chukar genes detected in red-legged (n = 43) and rock partridges (n = 4) of Spain, France and Italy as well as in either introduced (Italy) or native (Greece, Turkey) chukars (n = 35) were all from East Asia. Hence, a well-defined geographic origin of the exotic chukar genes polluting the genome of native Mediterranean A. rufa and A. graeca (inter-specific level) as well as A. chukar (intra-specific level), was demonstrated.






Similar content being viewed by others
Abbreviations
- CR:
-
Control Region
- Cyt-b :
-
Cytochrome-b gene
- MtDNA:
-
Mitochondrial DNA
- RAPD:
-
Random Amplified Polymorphic DNA
- STR:
-
Short Tandem Repeats
References
Abdusalyamov IA (1971) Fauna of Tajik SSR, vol. XIX. Part 1. Birds. Donish Press Dushanbe
Allendorf FW, Luikart G (2007) Conservation and the genetic of populations. Blackwell Publishing, Malden
Andreoli I (2006) Interspecific relationships among ciliated protozoa: a molecular approach. PhD dissertation, University of Pisa, Italy
Anttila CK, King RA, Ferris C et al (2000) Reciprocal hybrid formation of Spartina in San Francisco Bay. Mol Ecol 9:765–770
Barbanera F, Negro JJ, Di Giuseppe G et al (2005) Analysis of the genetic structure of red-legged partridge (Alectoris rufa, Galliformes) populations by means of mitochondrial DNA and RAPD markers: a study from central Italy. Biol Conserv 122:275–287
Barbanera F, Guerrini M, Hadjigerou P et al (2007) Genetic insight into Mediterranean chukar (Alectoris chukar, Galliformes) populations inferred from mitochondrial DNA and RAPD markers. Genetica 131:287–298
Barilani M, Bernard-Laurent A, Mucci N et al (2007) Hybridisation with introduced chukars (Alectoris chukar) threatens the gene pool integrity of native rock (A. graeca) and red-legged (A. rufa) partridge populations. Biol Conserv 137:57–69
Bernard-Laurent A (1984) Hybridation naturelle entre Perdrix bartavelle (Alectoris graeca saxatilis) et Perdrix rouge (Alectoris rufa) dans les Alpes Maritimes. Gibier Faune Sauvage 2:79–96
Buchan JC, Archie EA, Van Horn RC et al (2005) Locus effects and sources of error in non-invasive genotyping. Mol Ecol Notes 5:680–683
Clements JF (2007) The Clements checklist of the birds of the world, 6th edn. Cornell University, USA
Cottam C, Arnold LN, Saylor LW (1940) The chukar and Hungarian partridge in America. US Department Interior, Bio Survey, Wildlife Leaflets, BS-159, USA
Darling JA, Blum MJ (2007) DNA-based methods for monitoring invasive species: a review and prospectus. Biol Invasions 9:751–765
Dementiev GP, Gladkov NA, Isakov YA et al (1952) Rock or Chukar partridge. In: Dementev GP, Gladkov NA (eds) Birds of the soviet union, vol. 4. Sovetskaya Nauka, Moscow, pp 168–174
Dias D (1992) Rock (Alectoris graeca) and chukar (A. chukar) partridge introductions in Portugal and their possible hybridization with red-legged partridges (A. rufa): a research project. Gibier Faune Sauvage 9:781–784
Evanno G, Reganut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. EBO 1:47–50
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Frantz AC, Pope LC, Carpenter PJ et al (2003) Reliable microsatellite genotyping of the Eurasian badger (Meles meles) using faecal DNA. Mol Ecol 12:1649–1661
Frantzen MAJ, Silk JB, Ferguson JWH et al (1998) Empirical evaluation of preservation methods for faecal DNA. Mol Ecol 7:1423–1428
Fumihito A, Miyake T, Takada M et al (1995) The genetic link between the Chinese bamboo partridge (Bambusicola thoracica) and the chicken and junglefowls of the genus Gallus. Proc Natl Acad Sci USA 91:12505–12509
Garnier S, Alibert P, Audiot P et al (2004) Isolation by distance and sharp discontinuities in gene frequencies: implications for the phylogeography of an alpine insect species, Carabus soltieri. Mol Ecol 13:1883–1897
Gonzalez EG, Castilla AM, Zardoya R (2005) Novel polymorphic microsatellites for the red-legged partridge (Alectoris rufa) and cross-species amplification in Alectoris graeca. Mol Ecol Notes 5:449–451
Goodwin D (1986) Further notes on chukar and hybrid partridges in Britain and Europe. Avicult Mag 92:157–160
Guerrini M, Panayides P, Hadjigerou P et al (2007) Lack of genetic structure of Cypriot Alectoris chukar populations (Aves, Galliformes) as inferred from mtDNA sequencing data. ABC 30:105–114
Idaghdour Y, Broderick D, Korrida A (2003) Faeces as a source of DNA for molecular studies in a threatened population of great bustards. Conserv Genet 4:789–792
Kohn M, Knauer F, Stoffella A (1995) Conservation genetics of the European brown bear—a study using excremental PCR of nuclear and mitochondrial sequences. Mol Ecol 4:95–103
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Mack RN, Simberloff D, Lonsdale WM (2000) Biotic invasions: causes, epidemiology, global consequences and control. Ecol Appl 10:689–710
Madge S, McGowan P (2002) Pheasants, partridges and grouse. A and C Black Ltd., London
Maki-Petays H, Corander J, Aalto J et al (2007) No genetic evidence of sex-biased dispersal in a lekking bird, the capercaillie (Tetrao urogallus). J Evol Biol 20:865–873
Martinez-Fresno M, Henriques-Gil N, Arana P (2008) Mitochondrial DNA sequence variability in red-legged partridge, Alectoris rufa, Spanish populations and the origins of genetic contamination from A. chukar. Conserv Genet. doi:10.1007/s10592-007-9449-1
Ming M (2001) A checklist of the birds in Xingjian, China. Science Press, Beijing
Negro JJ, Torres MJ, Godoy JA (2001) RAPD analysis for detection and eradication of hybrid partridges (Alectoris rufa × A. graeca) in Spain. Biol Conserv 9:19–24
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of the AIC and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Randi E (1996) A mitochondrial cytochrome B phylogeny of the Alectoris partridges. Mol Phylogenet Evol 2:214–227
Randi E (2008) Detecting hybridization between wild species and their domesticated relatives. Mol Ecol 17:285–293
Randi E, Lucchini V (1998) Organization and evolution of the mitochondrial DNA control region in the Avian Genus Alectoris. J Mol Evol 47:449–462
Raymond M, Rousset F (1995) GENEPOP (version 3.1) is an update version of GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Regnaut S, Lucas FS, Fumagalli L (2006) DNA degradation in avian faecal samples and feasibility of non-invasive genetic studies of threatened capercaille populations. Conserv Genet 7:449–453
Rieseberg LH, Gerber D (1995) Hybridization in the Catalina Island mountain mahogany (Cercocarpus traskiae): RAPD evidence. Conserv Biol 9:199–203
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Scalera R (2001) Invasioni biologiche. Le introduzioni di vertebrati in Italia: un problema tra conservazione e globalizzazione. Collana Verde, vol. 103. Corpo Forestale dello Stato. Ministero delle Politiche Agricole e Forestali, Roma
Swofford DL (2002) PAUP*: Phylogenetic analysis using parsimony. Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts
Swofford DL, Olsen GJ, Waddel PJ et al (1996) Phylogenetic inference. In: Hillis DH, Moritz C, Bable BK (eds) Molecular systematics, 2nd edn. Sinauer Associates, Sunderland Massachusetts, pp 407–514
Tejedor MT, Monteagudo LV, Mautner S et al (2007) Introgression of Alectoris chukar genes into a Spanish wild Alectoris rufa population. J Hered 98:179–182
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
True GH (1937) The chukar partridge of Asia. Calif Fish Game 23:229–231
Vaha J-P, Primmer CR (2006) Efficiency of model-based Bayesian methods for detecting hybrid individuals under different hybridization scenarios and with different numbers of loci. Mol Ecol 15:63–72
Welsh J, McClelland M (1990) Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res 18:7213–7218
Williams JGK, Kubelik AR, Livak KJ et al (1990) DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Williams CL, Brust CR, Fendley TT et al (2005) A comparison of hybridization between Mottled Ducks (Anas fulgivula) and Mallards (A. platyrhynchos) in Florida and South Carolina using Microsatellite DNA Analysis. Conserv Genet 3:445–453
Yu FH, Yu FR, McGuire P et al (2004) Molecular phylogeny and biogeography of woolly flying squirrel (Rodentia: Sciuridae), inferred from mitochondrial cytochrome b gene sequences. Mol Phylogenet Evol 33:735–744
Zheng ZX (1987) A synopsis of the Avifauna of China. Science Press, Beijing
Acknowledgements
Authors are grateful to: F. Cappelli (Territorial Office for Biodiversity, Italian Forestry Service, Lucca), G. Luciani and M. Arrighi (Provincial Police, Livorno), R. Giombini (Italian Forestry Service, M. Marina) and their staff for the fieldwork on Elba Island; C. Pietri and R. Maupertuis (Fédération Départementale des Chasseurs de Haute-Corse, Bastia, France) for the A. rufa sampling in Corsica; J.J. Negro (Estacion Biológica de Doñana, Seville) and P. Prieto (Finca Lugar Nuevo, Andujar) for Spanish A. rufa samples; I. Andreoli and G. Petroni (Department of Biology, University of Pisa) for their valuable suggestions to set-up the snPCR. For the A. chukar samples we thank: P. Birtsas (Hunting Federation of Macedonia-Thrace, Greece), G. Arnellos and A. Sakoulis (Hunting Federation of Crete, Greece), P. Lemanis (Hunting Federation of Archipelago, Greece), C. Barboutis (Natural History Museum of Crete, Greece), E. Randi (INFS, Italy), I. Gursey (Turkey), Yerevan Zoo (Armenia), J. Saparmuradov (Institute of Desert, Flora and Fauna, Ashgabat, Turkmenistan), A.N. Ostaschenko (Institute for Biology and Pedology, Bishkek, Kyrgyzstan), J.I. Chaudhry (Afghanistan), N. Awan (Pakistan), O.V. Shilo (Novosibirsk Zoo, Russia), E. Burdinva (Perm Zoo, Russia), Y.A. Makhrov (Krasnoyarskpark of Flora and Fauna “Roev Ruchei”, Russia), A. Yedgebayeva (Almaty Zoo, Kazakhstan), L. Da (Institute of Biophysics, Beijing, China), Beijing Zoo (China), M. Ming (Xinjiang Institute of Ecology and Geography, Urumqi, China), N. Liu (School of Life Sciences, Lanzhou University, China), L. Gilbertson (Nevada Department of Wildlife, USA). We acknowledge S.I. Fokin (Biological Research Institute, St. Petersburg State University, Russia) for his help in contacting formerly USSR countries and L. Taglioli (Department of Biology, University of Pisa) for the statistical analysis. We are grateful to G. Grasseschi, C. Marchi, E. Mori and F.P. Frontini (Department of Biology, University of Pisa) for their qualified laboratory work. We thank the University of Washington Burke Museum (Seattle, USA) and the Natural History Museum of Crete (Heraklion, Greece) for the A. chukar tissues loan. This work was supported by grants of the Provincia di Livorno, INTERREG III Toscana—Corsica—Sardegna and the Cypriot Game Fund Service, Ministry of Interior, Nicosia, Cyprus.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbanera, F., Guerrini, M., Khan, A.A. et al. Human-mediated introgression of exotic chukar (Alectoris chukar, Galliformes) genes from East Asia into native Mediterranean partridges. Biol Invasions 11, 333–348 (2009). https://doi.org/10.1007/s10530-008-9251-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-008-9251-0