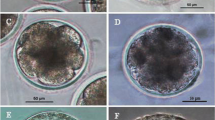
Abstract
Objective
The aim of this study was to investigate the developmental competence of oocytes parthenogenetically activated by an electric pulse (EP) and treated with anisomycin and to determine whether this method is applicable to somatic cell nuclear transfer (SCNT).
Results
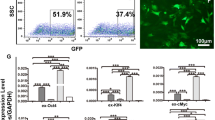
Embryos derived from porcine oocytes parthenogenetically activated by an EP and treatment with 0.01 µg/mL anisomycin had a significantly improved in vitro developmental capacity. Furthermore, 66.6% of blastocysts derived from these embryos had a diploid karyotype. The blastocyst formation rate of cloned embryos was similar between oocytes activated by an EP and treated with 2 mM 6-dimethylaminopurine for 4 h and those activated by an EP and treated with 0.01 µg/mL anisomycin for 4 h. The level of maturation-promoting factor was significantly decreased in oocytes activated by an EP and treated with anisomycin. Finally, the mRNA expression levels of apoptosis-related genes (Bax and Bcl-2) and pluripotency-related genes (Oct4, Nanog, and Sox2) were checked by RT-PCR.
Conclusion
Our results demonstrate that porcine oocyte activation via an EP in combination with anisomycin treatment can lead to a high blastocyst formation rate in parthenogenetic activation and SCNT experiments.




Similar content being viewed by others
References
Bhak JS, Lee SL, Ock SA et al (2006) Developmental rate and ploidy of embryos produced by nuclear transfer with different activation treatments in cattle. Anim Reprod Sci 92:37–49
Blelloch R, Wang Z, Meissner A et al (2006) Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells 24:2007–2013
Choi I, Campbell KHS (2015) The role of protein kinases in reprogramming and development of SCNT embryos. J Embryo Transf 30:33–43
Cui MS, Liu ZX, Wang XL et al (2011) Relationship between differential expression of Bax and Bcl-2 genes and developmental differences of porcine parthenotes cultured in PZM-3 and NCSU-23. Agric Sci China 10:1772–1780
De LFR, King WA (1998) Developmental consequences of karyokinesis without cytokinesis during the first mitotic cell cycle of bovine parthenotes. Biol Reprod 58(4):952–962
Dinnyés A, Hirao Y, Nagai T (1999) Parthenogenetic activation of porcine oocytes by electric pulse and/or butyrolactone I treatment. Cloning 1:209–216
Felmer R, Arias ME (2015) Activation treatment of recipient oocytes affects the subsequent development and ploidy of bovine parthenogenetic and somatic cell nuclear transfer (SCNT) embryos. Mol Reprod Dev 82:441–449
Han B, Gao J (2013) Effects of chemical combinations on the parthenogenetic activation of mouse oocytes. Exp Ther Med 5:1281–1288
Hao YH et al (2005) Developmental competence of porcine parthenogenetic embryos relative to embryonic chromosomal abnormalities. Mol Reprod Dev 1:77–82
Koo DB, Chae JI, Kim JS et al (2005) Inactivation of MPF and MAP kinase by single electrical stimulus for parthenogenetic development of porcine oocytes. Mol Reprod Dev 72:542–549
Kubiak JZ, Weber M, De PH et al (1993) The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. EMBO J 12:3773–3778
Lee HS, Kim KH, Kim EY et al (2016) Obox4-silencing-activated STAT3 and MPF/MAPK signaling accelerate nuclear membrane breakdown in mouse oocytes. Reproduction 151:369–378
Liu L, Ju JC, Yang X (1998) Differential inactivation of maturation-promoting factor and mitogen-activated protein kinase following parthenogenetic activation of bovine oocytes. Biol Reprod 59:537–545
Milazzotto M, Feitosa W, Coutinho A et al (2008) Effect of chemical or electrical activation of bovine oocytes on blastocyst development and quality. Reprod Domest Anim 43:319–322
Nanassy L, Lee K, Javor A et al (2007) Changes in MPF and MAPK activities in porcine oocytes activated by different methods. Theriogenology 68:146–152
Ostrup E, Hyttel P, Ostrup O (2011) Embryo-maternal communication: signalling before and during placentation in cattle and pig. Reprod Fert Dev 23:964–975
Presicce GA, Yang X (1994) Parthenogenetic development of bovine oocytes matured in vitro for 24 hr and activated by ethanol and cycloheximide. Mol Reprod Dev 38:380–385
Sepulveda-Rincon LP, Solanas ED, Serrano-Revuelta E et al (2016) Early epigenetic reprogramming in fertilized, cloned, and parthenogenetic embryos. Theriogenology 86:91–98
Sommer CA, Stadtfeld M, Murphy GJ et al (2009) Induced pluripotent stem cell generation using a single Lentiviral stem cell cassette. Stem Cells 27:543–549
Szöllösi MS, Kubiak JZ, Debey P et al (1993) Inhibition of protein kinases by 6-dimethylaminopurine accelerates the transition to interphase in activated mouse oocytes. J Cell Sci 104:861–872
Velde AVD, Liu L, Bols PEJ et al (1999) Cell allocation and chromosomal complement of parthenogenetic and IVF bovine embryos. Mol Reprod Dev 54:57–62
Whitaker M (1996) Control of meiotic arrest. Rev Reprod 1:127–135
Yin XJ, Tani T, Yonemura I et al (2002) Production of cloned pigs from adult somatic cells by chemically assisted removal of maternal chromosomes. Biol Reprod 67:442–446
Acknowledgements
This work was supported by the State Key Development Program for Basic Research of China (Grant No. 20150622005JC) and the Institute for Basic Science (Grant No. IBS-R021-D1-2015-a02).
Supporting information
Supplementary Table 1—Primer sequences used for gene expression analysis.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yu-Chen Zhang, Long Jin, Hai-Ying Zhu have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, YC., Jin, L., Zhu, HY. et al. The developmental competence of oocytes parthenogenetically activated by an electric pulse and anisomycin treatment. Biotechnol Lett 39, 189–196 (2017). https://doi.org/10.1007/s10529-016-2249-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2249-2