Abstract
Objectives
To find a simple enzymatic strategy for the efficient synthesis of the expensive 5′-hydroxyomeprazole sulfide, a recently identified minor human metabolite, from omeprazole sulfide, which is an inexpensive substrate.
Results
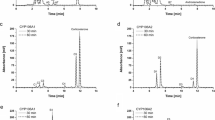
The practical synthetic strategy for the 5′-OH omeprazole sulfide was accomplished with a set of highly active CYP102A1 mutants, which were obtained by blue colony screening from CYP102A1 libraries with a high conversion yield. The mutant and even the wild-type enzyme of CYP102A1 catalyzed the high regioselective (98 %) C-H hydroxylation of omeprazole sulfide to 5′-OH omeprazole sulfide with a high conversion yield (85–90 %).
Conclusions
A highly efficient synthesis of 5′-OH omeprazole sulfide was developed using CYP102A1 from Bacillus megaterium as a biocatalyst.





Similar content being viewed by others
References
Butler CF, Peet C, Mason AE, Voice MW, Leys D, Munro AW (2013) Key mutations alter the cytochrome P450 BM3 conformational landscape and remove inherent substrate bias. J Biol Chem 288:25387–25399
Butler CF, Peet C, McLean KJ, Baynham MT, Blankley RT, Fisher K, Rigby SE, Leys D, Voice MW, Munro AW (2014) Human P450-like oxidation of diverse proton pump inhibitor drug by ‘gatekeeper’ mutants of flavocytochrome P450 BM3. Biochem J 460:247–259
Li XQ, Weidolf L, Simonsson R, Andersson TB (2005) Enantiomer/enantiomer interactions between the S- and R-isomers of omeprazole in human cytochrome P450 enzymes: major role of CYP2C19 and CYP3A4. J Pharmacol Exp Ther 315:777–787
Nevado JJ, Peñalvo GC, Dorado RM, Robledo VR (2014) Simultaneous determination of omeprazole and their main metabolites in human urine samples by capillary electrophoresis using electrospray ionization-mass spectrometry detection. J Pharm Biomed Anal 92:211–219
Obach RS (2013) Pharmacologically active drug metabolites: impact on drug discovery and pharmacotherapy. Pharmacol Rev 65:578–640
Rezk NL, Brown KC, Kashuba AD (2006) A simple and sensitive bioanalytical assay for simultaneous determination of omeprazole and its three major metabolites in human blood plasma using RP-HPLC after a simple liquid–liquid extraction procedure. J Chromatogr B 844:314–321
Ryu SH, Park BY, Kim SY, Park SH, Jung HJ, Park M, Park KD, Ahn T, Kang HS, Yun CH (2014) Regioselective hydroxylation of omeprazole enantiomers by bacterial CYP102A1 mutants. Drug Metab Dispos 42:1493–1497
Saccar CL (2009) The pharmacology of esomeprazole and its role in gastric acid related diseases. Expert Opin Drug Metab Toxicol 5:1113–1124
van Vugt-Lussenburg BM, Damsten MC, Maasdijk DM, Vermeulen NP, Commandeur JN (2006) Heterotropic and homotropic cooperativity by a drug-metabolising mutant of cytochrome P450 BM3. Biochem Biophys Res Commun 346:810–818
Wedemeyer RS, Blume H (2014) Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf 10:187–194
Whitehouse CJ, Bell SG, Wong LL (2012) P450 BM3 (CYP102A1): connecting the dots. Chem Soc Rev 41:1218–1260
Yun CH, Yim SK, Kim DH, Ahn T (2006) Functional expression of human cytochrome P450 enzymes in Escherichia coli. Curr Drug Metab 7:411–429
Yun CH, Kim KH, Kim DH, Jung HC, Pan JG (2007) The bacterial P450 BM3: a prototype for a biocatalyst with human P450 activities. Trends Biotechnol 25:289–298
Acknowledgments
This research was supported by the National Research Foundation of Korea (Grant NRF-2016R1A2B4006978) and by the Next-Generation BioGreen 21 program (SSAC Grant# PJ011058), Rural Development Administration, Republic of Korea.
Supporting information
Supplementary Methods-Generation of the CYP102A1 libraries.
Supplementary Methods-Screening of the CYP102A1 libraries.
Supplementary Methods-Liquid chromatography-mass spectrometry analysis.
Supplementary Methods-Identification of the omeprazole sulfide metabolite by NMR spectroscopy.
Supplementary Table 1. Mutated amino acid residues of the CYP102A1 mutants used in this study.
Supplementary Table 2. 1H chemical shifts for 5'-OH omeprazole sulfide.
Supplementary Figure 1. Metabolic pathway of omeprazole in humans.
Supplementary Figure 2. Screening of CYP102A1 mutant libraries.
Supplementary Figure 3. Close look at the 3.81 ppm and NOE results of 5′-OH omeprazole sulfide.
Supplementary Figure 4. The mutation sites for CYP102A1 M16V2 and its mutants.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jang, HH., Ryu, SH., Le, TK. et al. Regioselective C-H hydroxylation of omeprazole sulfide by Bacillus megaterium CYP102A1 to produce a human metabolite. Biotechnol Lett 39, 105–112 (2017). https://doi.org/10.1007/s10529-016-2211-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2211-3