Abstract
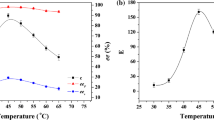
The solvent effects of cyclopentyl methyl ether (CPME) on the reaction rates and enzyme enantioselectivity in the enantioselective transesterifications of racemic 6-methyl-5-hepten-2-ol (racemic sulcatol: SUL) and racemic 2,2-dimethyl-1,3-dioxolane-4-methanol (racemic solketal: SOL) with a series of enol esters catalyzed by Pseudomonas cepacia lipase co-lyophilized with cyclodextrins (α-, β-, γ-, partially methylated β-,␣and 2,3,6-tri-O-methyl-β-cyclodextrin: αCyD; βCyD; γCyD; Me1.78 βCyD; Me3βCyD) were investigated and compared with those in diisopropyl ether (IPE). In the case of SUL, enzyme activities of the co-lyophilizate with Me1.78 βCyD in CPME were lower than those in IPE with every acyl source, however, the absolute enantiopreference was shown in the transesterification with vinyl butyrate (VBR) in IPME. When the substrates were SOL and VBR, the enzyme activities in CPME were greatly enhanced as high as 1.6–9.8-fold, while the enantioselectivities in CPME were comparable to those in IPE.
Similar content being viewed by others
References
G Carre G Ottolina S Riva (1995) ArticleTitleRole of solvents in the control of enzyme selectivity in organic media Trends Biotechnol. 13 63–70
C-S Chen Y Fujimoto G Girdaukas CJ Sih (1982) ArticleTitleQuantitative analyses of biochemical kinetic resolutions of enantiomer J. Am. Chem. Soc. 104 7294–7299
C-S Chen CJ Sih (1989) ArticleTitleGeneral aspect and optimization of enantioselective biocatalysis in organic solvent: the use of lipases Angew. Chem. Int. Ed. Engl. 28 695–707
Y Hirose K Kariya I Sakai Y Kurono H Ebiike K Achiwa (1992) ArticleTitleDrastic solvent effect on lipase-catalyzed enantioselective hydrolysis of prochiral 1,4-dihydropyridines Tetrahedron Lett. 33 7157–7160
Y Hirose K Kariya Y Nakanishi Y Kurono K Achiwa (1995) ArticleTitleInversion of enantioselectivity in hydrolysis of 1,4-dihydropyridines by point mutation of lipase PS Tetrahedron Lett. 36 1063–1066
E Holmberg K Hult (1991) ArticleTitleTemperature as an enantioselective parameter in enzymatic resolutions of racemic mixtures Biotechnol. Lett. 13 323–326
BR Johnston B Morgan AC Oehlschlager S Ramaswamy (1991) ArticleTitleA convenient synthesis of both enantiomers of seudenol and their conversion to 1-methyl-2-cyclohexen-l-ol Tetrahedron: Asymmetry 2 377–380
DA Lang BW Dijkstra (1998) ArticleTitleStructural investigations of the regio- and enantioselectivity of lipases Chem. Phys. Lipids 93 115–122
AL Margolin (1993) ArticleTitleEnzymes in the synthesis of chiral drugs Enzyme Microb. Technol. 15 267–280
Y Mine K Fukunaga M Yoshimoto K Nakao Y Sugimura (2001) ArticleTitleModification of lipases with poly(ethylene glycol) and poly(oxyethylene) detergents and their catalytic activities in organic solvents J. Biosci. Bioeng. 92 539–543
Y Mine K Fukunaga K Itoh M Yoshimoto K Nakao Y Sugimura (2003) ArticleTitleEnhanced enzyme activity and enantioselectivity of lipases in organic solvents by crown ethers and cyclodextrins J. Biosci. Bioeng. 95 441–47
K Mori BG Hazra RJ Pfeiffer AK Gupta BS Lindgren (1987) ArticleTitleSynthesis and bioactivity of optically-active forms of 1-methyl-2-cyclohexene-l-ol, an aggregation pheromone of Dendroctonus pseudotsugae Tetrahedron 43 2249–2254
K Nakamura Y Takabe T Kitayama Ohno A (1991) ArticleTitleEffect of solvent structure on enantioselectivity of lipase-catalyzed transesterification Tetrahedron Lett. 32 4941–4944
S Parida JS Dordick (1991) ArticleTitleSubstrate structure and solvent hydrophobicity control lipase catalysis and enantioselectivity in organic media J. Am. Chem. Soc. 113 2253–2259
T Sakai T Kishimoto Y Tanaka T Ema M Utaka (1998) ArticleTitleLow-temperature method for enhancement of enantioselectivity in the lipase-catalyzed kinetic resolution of solketal and some chiral alcohols Tetrahedron Lett. 39 7881–7884
T Sakurai AL Margolin AJ Russell AM Klibanov (1988) ArticleTitleControl of enzyme enantioselectivity by the reaction medium J. Am. Chem. Soc. 110 7236–7237
E Santaniello P Ferraboschi P Grisenti (1993) ArticleTitleLipase-catalyzed transesterification in organic solvents: applications to the preparation of enantiomerically pure compounds Enzyme Microb. Technol. 15 367–382
F Secundo S Riva G Carrea (1992) ArticleTitleEffect of medium and reaction conditions on the enantioselectivity of lipases in organic solvents and possible rationales Tetrahedron: Asymmetry 3 267–280
CJ Sih G Girdauks C-S Chen JC Sih (1996) Enzymatic resolutions of alcohols, esters, and nitrogen-containing compounds AMP Koskinen M Klibanov (Eds) Enzymatic Reactions in Organic Media Blackie Academic & Professional Glasgow 94–139
K Watanabe K Goto (2003) ArticleTitleCyclopentyl methyl ether (CAS No. 5614-37-9) Yuki Gosei Kagaku Kyokaishi 61 807–808
CR Wescott AM Klibanov (1994) ArticleTitleThe solvent dependence of enzyme specificity Biochim. Biophys. Acta 1206 1–9
Author information
Authors and Affiliations
Corresponding author
Additional information
Revisions requested 16 December 2004; Revisions received 17 January 2005
Rights and permissions
About this article
Cite this article
Mine, Y., Zhang, L., Fukunaga, K. et al. Enhancement of enzyme activity and enantioselectivity by cyclopentyl methyl ether in the transesterification catalyzed by Pseudomonas cepacia lipase co-lyophilized with cyclodextrins. Biotechnol Lett 27, 383–388 (2005). https://doi.org/10.1007/s10529-005-1527-1
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10529-005-1527-1