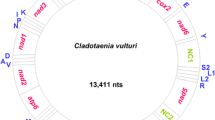
Hox genes are important in forming the anterior-posterior body axis pattern in the early developmental stage of animals. The conserved nature of the genomic organization of Hox genes is well known in diverse metazoans. To understand the Hox gene architecture in human-infecting Taenia tapeworms, we conducted a genomic survey of the Hox gene using degenerative polymerase chain reaction primers in Taenia asiatica. Six Hox gene orthologs from 276 clones were identified. Comparative analysis revealed that T. asiatica has six Hox orthologs, including two lab/Hox1, two Hox3, one Dfd/Hox4, and one Lox2/Lox4. The results suggest that Taenia Hox genes may have undergone independent gene duplication in two Hox paralogs. The failure to detect Post1/2 orthologs in T. asiatica may suggest that sequence divergence or the secondary loss of the posterior genes has occurred in the lineage leading to the cestode and trematode.

Similar content being viewed by others
REFERENCES
Balavoine, G. (1996). Identification of members of several homeobox genes in a planarian using a ligation-mediated polymerase chain reaction technique. Nucleic Acids Res. 24:1547–1553.
Balavoine, G., and Telford, M. J. (1995). Identification of planarian homeobox sequences indicates the antiquity of most Hox/homeotic gene subclasses. Proc. Natl. Acad. Sci. USA 92:7227–7231.
Bartels, J. L., Murtha, M. T., and Ruddle, F. H. (1993). Multiple Hox/HOM-Class Homeoboxes in Platyhelminthes. Mol. Phylogenet. Evol. 2:143–151.
Bayascas, J. R., Castillo, E., Munoz-Marmol, A. M., and Salo, E. (1997). Planarian Hox genes: Novel patterns of expression during regeneration. Development 124:141–148.
Callaerts, P., Lee, P. N., Hartmann, B., Farfan, C., Choy, D. W., Ikeo, K., Fischbach, K. F., Gehring, W. J., and de Couet, H. G. (2002). HOX genes in the sepiolid squid Euprymna scolopes: Implications for the evolution of complex body plans. Proc. Natl. Acad. Sci. USA 99:2088–2093.
Cho, S. J., Cho, P. Y., Lee, M. S., Hur, S. Y., Lee, J. A., Kim, S. K., Koh, K. S., Na, Y. E., Choo, J. K., Kim, C. B., and Park, S. C. (2003). Hox genes from the earthworm Perionyx excavatus. Dev. Genes Evol. 213:207–210.
De Rosa, R., Grenier, J. K., Andreeca, T., Cook, C. E., Adoutte, A., Akam, M., Carroll, S. B., and Balavoine, G. (1999). Hox genes in brachiopods and priapulids and protostome evolution. Nature 399:772–776.
Eom, K. S., and Rim, H. J. (1993). Morphological descriptions of Taenia asiatica sp. Korean J. Parasitol. 31:1–6.
Garcia-Fernandez, J., Baguna, J., and Salo, E. (1991). Planarian homeobox genes: Cloning, sequence analysis, and expression. Proc. Natl. Acad. Sci. USA 88:7338–7342.
Gehring, W. J. (1987). Homeoboxes in the study of development. Science 236:1245–1252.
Irvine, S. Q., Warinner, S. A., Hunter, J. D., and Martindale, M. Q. (1997). A survey of homeobox genes in Chaetopterus variopedatus and analysis of polychaete homeodomains. Mol. Phylogenet. Evol. 7:331–345.
McGinnis, W., and Krumlauf, R. (1992). Homeobox genes and axial patterning. Cell 68:283–302.
Misof, B. Y., and Wagner, G. P. (1996). Evidence for four Hox clusters in the killifish Fundulus heteroclitus (teleostei). Mol. Phylogenet. Evol. 5:309–322.
Mito, T., and Endo, K. (2000). PCR survey of Hox genes in the crinoid and ophiuroid: Evidence for anterior conservation and posterior expansion in the echinoderm Hox gene cluster. Mol. Phylogenet. Evol. 14:375–388.
Murtha, M. T., Lechman, J. F., and Ruddle, F. H. (1991). Detection of homeobox genes in development and evolution. Proc. Natl. Acad. Sci. USA 88:10711–10715.
Nogi, T., and Watanabe, K. (2001). Position-specific and non-colinear expression of the planarian posterior (Abdominal-B-like) gene. Dev. Growth Differ. 43:177–184.
Orii, H., Kato, K., Umesono, Y., Sakurai, T., Agata, K., and Watanabe, K. (1999). The planarian HOM/HOX homeobox genes (Plox) expressed along the anteroposterior axis. Dev. Biol. 210:456–468.
Papillon, D., Perez, Y., Fasano, L., Parco, Y. L., and Caubit, X. (2003). Hox gene survey in the chaetognath Spadella cephaloptera: Evolutionary implications. Dev. Genes Evol. 213:142–148.
Pierce, R. J., Wu, W., Hirai, H., Ivens, A., Murphy, L. D., Noel, C., Johnston, D. A., Artiguenave, F., Adams, M., Cornette, J., Viscogliosi, E., Capron, M., and Balavoine, G. (2005). Evidence for a dispersed Hox gene cluster in the platyhelminth parasite Schistosoma mansoni. Mol. Biol. Evol. 22:2491–2503.
Scott, M. P., Tamkun, J. W., and Hartzell, G. W. (1989). The structure and function of the homeodomain. Biochim. Biophys. Acta 989:25–48.
Snow, P., and Buss, L. W. (1994). HOM/Hox-type homeoboxes from Stylaria lacustris (Annelida: Oligochaeta). Mol. Phylogenet. Evol. 3:360–364.
Telford, M. J. (2000). Turning Hox ``signatures'' into synapomorphies. Evol. Dev. 2:360–364.
Thompson, J. D., Gibson, T. J., Plewniak, F., and Higgins, D. G. (1997). The Clustal X Windows interface flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882.
ACKNOWLEDGMENTS
Nucleotide sequence data reported in this paper are available in the GenBank, EMBL, and DDBJ databases under accession nos. DQ069784 and DQ387457-387461.
This work was supported by the KRIBB Research Initiative Program and the Bioinfrastructure Program of the Korea Ministry of Science and Technology. Parasite materials used in this study were provided by the Parasite Resource Bank of Korea National Research Resource Center (R21-2005-000-10007-0), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KH., Lee, Y.S., Jeon, HK. et al. Hox Genes from the Tapeworm Taenia asiatica (Platyhelminthes: Cestoda). Biochem Genet 45, 335–343 (2007). https://doi.org/10.1007/s10528-007-9078-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-007-9078-x