Abstract
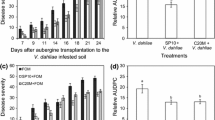
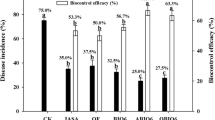
Fusarium wilt of watermelon commonly occurs in locations where the crop has been grown for many seasons. Its occurrence results in a severely decreased watermelon crop. The goal of this study was to assess the capability of a new product (bio-organic fertilizer) to control the wilt in Fusarium-infested soil. Pot experiments were conducted under growth chamber and greenhouse conditions. The results showed that the fertilizer controlled the wilt disease. Compared with control pots, the incidence rates of Fusarium wilt at 27 and 63 days following treatment of the plants with the bio-organic fertilizer at a rate of 0.5% (organic fertilizer + antagonistic microorganisms, including 3 × 109 CFU g−1 Paenibacillus polymyxa and 5 × 107 CFU g−1 Trichoderma harzianum) were reduced by 84.9 and 75.0%, respectively, in both the growth chamber and greenhouse settings. The activities of antioxidases (catalase, superoxide dismutase and peroxidase) in watermelon leaves increased by 38.9, 150 and 250%, respectively. In the roots, stems and leaves, the activity of β-1,3-glucanase (pathogenesis-related proteins) increased by 80, 1140 and 100% and that of chitinase increased by 240, 80, and 20%, respectively, while the contents of malondialdehyde fell by 56.8, 42.1 and 45.9%, respectively. These results indicate that this new fertilizer formula is capable of protecting watermelon from Fusarium oxysporum f.sp. niveum. The elevated levels of defense-related enzymes are consistent with the induction and enhancement of systemic acquired resistance of plant.









Similar content being viewed by others
Abbreviations
- BOF:
-
Bio-organic fertilizer
- CAT:
-
Catalase
- FON:
-
Fusarium oxysporum f.sp.nevium
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- OF:
-
Organic fertilizer
- POD:
-
Guaiacol peroxidase
- SOD:
-
Superoxide dismutase
References
Abeles FB, Forrence LE (1970) Temporal and hormonal control of β-1,3-glucanase in Phaseolus vulgaris L. Plant Physiol 45:395–400
Abeles FB, Bosshart RP, Fortence LF (1970) Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol 47:129–134
Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2007) A role for brassinosteroids in the amelioration of aluminum stress through antioxidant system in mung bean. Environ Exp Bot 62:53–159
Armstrong GM, Armstrong JK (1981) Formae speciales and races of Fusarium oxysporum causing wilt disease. In: Nelson PE, Toussoun TA, Cook RJ (eds) Fusarium: disease, biology, and taxonomy. Pennsylvania State University Press, Philadelphia, pp 156–185
Ash C, Priest FG, Collins MD (1994) Molecular identification of rRNA group 3 Bacillus PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie van Leeuwen Hoek 64:253–260
Bartniki-Garcia S (1968) Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol 22:87–108
Booth C (1971) The genus Fusarium. The Eastern Press, London, pp 147–149
Burge MN (1988) The scope of fungi in biological control. In: Burge MN (ed) Fungi in biological control systems. Manchester University Press, Manchester, pp 1–18
Chet A, Benhamou N, Haran S (1998) Mycoparasitism and lytic enzymes. In: Harman GA, Kubicek C (eds) Trichoderma and Gliocladium: enzymes, biological control and commercial applications, vol 2. Taylor and Francis, London, pp 153–172
Claire S-W, Houot S, Claude A (1996) Increased soil suppressiveness to Fusarium wilt of flax after addition of municipal solid waste compost. Soil Biol Biochem 28:1207–1214
Cook RJ (1990) In: Hornby D (ed) Biological control of soil-borne plant pathogens. CAB Int/Redwood Press, Wallingford/Wiltshire, pp 1–14
Cook RJ (1993) Making greater use of introduced microorganisms for biological control of plant pathogens. Annu Rev Phytopathol 31:53–80
Cotxarrera L, Trillas-Gay MI, Steinberg C, Alabouvette C (2001) Use of sewage sludge compost and Trichoderma asperellum isolates to suppress Fusarium wilt of tomato. Soil Biol Biochem 34:467–476
Dijksterhuis J, Sanders M, Gorris LG, Smid EJ (1999) Antibiosis plays a role in the context of direct interaction during antagonism of Paenibacillus polymyxa towards Fusarium oxysporum. J Appl Microbiol 86:13–21
Domsch KH, Games W (1972) Fungi in agricultural soils (translated by Hudson PS). Longman Group, London, pp 67–78
Dubey SC, Suresh M, Singh B (2007) Evaluation of Trichoderma species against Fusarium oxysporum f.sp. ciceris for integrated management of chickpea wilt. Biol Control 40:118–127
Duijff BJ, Recorbet G, Peter A, Bakker HM, Loper JE, Lemanceau P (1999) Microbial antagonism at the root level is involved in the suppression of Fusarium wilt by the combination of nonpathogenic Fusarium oxysporum Fo47 and Pseudomonas putida WCS358. Phytopathol 89:1073–1079
Fink W, Liefland M, Mendgen K (1988) Chitinases and β-1,3-glucanases in the apoplastic compartment of oat leaves (Avena sativa L.). Plant Physiol 88:270–285
Fulton HR (1927) Organic fertilizers and cotton wilt control. Science 66:193–194
Garcia-Limones C, Hervas A, Navas-Cortes JA, Jimenez-Diaz RM, Tena M (2002) Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f.sp. ciceris. Physiol Mol Plant Pathol 61:325–337
Gerlach W, Nirenberg H (1982) The genus Fusarium: a pictorial atlas. Kommissionsverlag Paul Parey, Berlin, pp 347–349
Hartman GL, Huang YH, Li S (2004) Phytotoxicity of Fusarium solani culture filtrates from soybeans and other hosts assayed by stem cuttings. Australas Plant Pathol 33:9–24
Hervas A, Linda B, Jimenez-Diaz RM (1997) Influence of chickpea genotype and Bacillus sp on protection from Fusarium wilt by seed treatment with nonpathogenic Fusarium oxysporum. Eur J Plant Pathol 103:631–642
Hervas A, Linda B, Datnoff LE, Jimenez-Diaz RM (1998) Effect of commercial and indigenous microorganisms on Fusarium wilt development in chickpea. Biol Control 13:166–176
Hiroshi Y, Yoshinobu H, Sano H (2006) Polyamine oxidase is one of the key elements for oxidation burst to induce programmed cell death in tobacco cultured cells. Plant Physiol 142:193–206
Idris A, Abuschagne LHN, Korsten L (2007) Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biol Control 40:97–106
Joffe AZ (1986) Fusarium species: their biology and toxicology. Wiley, New York
Kajimura Y, Kaneda M (1996) Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8 taxonomy, fermentation, isolation structure elucidation and biological activity. J Antibiot 49:129–135
Kavroulakisa N, Ehaliotisa C, Ntougiasa S, Zervakisa GI, Papadopoulou KK (2005) Local and systemic resistance against fungal pathogens of tomato plants elicited by a compost derived from agricultural residues. Physiol Mol Plant Pathol 66:163–174
Khan Z, Kim SG, Jeon YH, Khan HU, Son SH, Kim YH (2008) A plant growth promoting rhizobacterium, Paenibacillus polymyxa strain GBR-1, suppress root-knot nematode. Bioresour Technol 99:3016–3023
Kharbanda PD, Yang J, Beatty P, Jensen S, Tewari JP (1999) Biocontrol of Leptospheria maculans and other pathogens of canola with Paenibacillus polymyxa PKB1. In: Proc 10th Int Rapeseed Congress. Canberra, Australia
Kim YK (1995) Biological control of phytophthora blight of red pepper by antagonistic Bacillus polymyxa AC-1. PhD thesis. Seoul National University, Seoul
Komada H (1990) Biological control of Fusarium wilts in Japan. In: Hornby D (ed) Biological control of soil-borne plant pathogens. CAB Int/Redwood Press, Wallingford/Wiltshire, pp 65–75
Krauss U, Soberanis W (2002) Effect of fertilization and biocontrol application frequency on cocoa pod diseases. Biol Control 24:82–89
Kuc J (1990) Immunization for the control of plant disease. In: Hornby D (ed) Biological control of soil-borne plant pathogens. CAB Int/Redwood Press, Melksham, pp 355–369
Leonardo D, Blanca LF, Landa B, Weller DM (2006) Host crop affects rhizosphere colonization and competitiveness of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology 96:751–762
Manoranjan K, Dinabandhu M (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Martyn RD (1996) Fusarium wilt of watermelon. In: Zitter TA, Hopkins DL, Thomas CA (eds) Compendium of cucurbit diseases. The American Phytopathology Society, St. Paul, pp 13–14
Papavizas GC (1985) Trichoderma and Gliocladium: biology, ecology and potential for biocontrol. Annu Rev Phytopathol 23:23–54
Papavizas GC, Lewis JA (1988) The use of fungi in integrated control of plant diseases. In: Burge MN (ed) Fungi in biological control systems. Manchester University Press, Manchester, pp 235–253
Park CS, Paulitz TC, Baker R (1988) Biocontrol of Fusarium wilt of cucumber resulting from interactions between Pseudomonas putida and non-pathogenic isolates of Fusarium oxysporum. Phytopathology 78:190–194
Pegg GF, Vesset JC (1973) Chitinase activity in Lycopersicon esculentum and its relationship to the in vivo lyses of Verticillium alboatrum mycelium. Physiol Plant Pathol 3:207–222
Rojo FG, Reynoso MM, Ferez M, Chulze SN, Torres AM (2007) Biological control by Trichoderma species of Fusarium solani causing peanut crown root rot under field conditions. Crop Protect 26:549–555
Sabuquillo P, Cal AD, Melgarejo P (2006) Biocontrol of tomato wilt by Panicillium oxalicum preparations in different crop conditions. Biol Control 37:256–265
Saravanan T, Muthusamy M, Marimuthu T (2003) Development of integrated approach to manage the fusarial wilt of banana. Crop Protect 22:1117–1123
Schraudner M, Ernst D, Langebartels C, Sandermann H (1992) Biochemical plant responses to ozone III. Activation of the defense-related proteins β-1,3-glucanase and chitinase in tobacco leaves. Plant Physiol 99:1321–1328
Schrith MN, Becker JO (1990) Concepts of ecological and physiological activities of rhizobacteria related to biological control and plant growth promotion. In: Hornby D (ed) Biological control of soil-borne plant pathogens. CAB Int/Redwood Press, Melksham, pp 391–397
Smith J, Hammerschmidt R (1988) Comparative study of acidic peroxidases associated with induced resistance in cucumber, muskmelon and watermelon. Physiol Mol Plant Pathol 33:255–261
Tawfic AA, Allam ADA (2004) Improving cumin production under soil infestation with Fusarium wilt pathogen: II field trial of different landraces and seed treatments. Assoc Univ Bull Environ Res 7:47–63
Termoshuizen AJ, Rijn EV, Gaag DJVD, Alabouvette C, Chen Y, Lagerlof J, Malandrakis AA, Paplomatas EJ, Ramert B, Ryckeboer J, Steinberg C, Zmora-Nahum S (2006) Suppressiveness of 18 composts against 7 pathosystems: variability in pathogen response. Soil Biol Biochem 38:2461–2477
Trillas MI, Casanova E, Corxarrera L, Ordovas J, Borrero C, Aviles M (2006) Composts from agricultural waste and the Trichoderma asperellum strain T-34 suppress Rhizoctonia solani in cucumber seedlings. Biol Control 39:32–38
Tu CC, Chang YH (1983) Soil microbial activity in relation to Fusarium wilt suppression soils and conducive soil. In: Proc Republic of China–Federal Republic of Germany Seminar Plant Nutrition Soil Sciences. National Science Council, Taipei, pp 189–196
Tukaj Z, Bascik-Remisiewicz A, Skowronski T, Tukaj C (2007) Cadmium effect on the growth, photosynthesis, ultrastructure and phytochelatin content of green microalga Scenedesmus armatus: A study at low and elevated CO2 concentration. Environ Exp Bot 60:291–299
Utkhede RS (2006) Soil sickness, replant problem or replant disease and its integrated control. Allelop J 18:23–38
Vernay P, Gauthier-Moussard C, Hitmi A (2007) Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 68:1563–1575
Whipps JM, Lewis K, Cooke RC (1988) Mycoparasitism and plant disease control. In: Burge MN (ed) Fungi in biological control systems. Manchester University Press, Manchester, pp 161–187
Acknowledgements
We gratefully acknowledge the financial support from the Science and Technology Ministry of China on project for Basic research on fertilizer saving and efficiency improvement for sustainable utilization of farmland (2007CB109304, 2006GB23600454, 2006AAD10Z416 and 2006BAD10B09) and the Agricultural Ministry of China (2006-G62 and 06-07-04B). We would like to thank Professor Frank White, Kansas State University, and Dr. Scott Johnson, Scottish Crop Research Institute, for their warmhearted and careful correction for this manuscript. We would also like to thank Dr. Xiaoyu Wang and Dr. Jiahuan Zhang, both from the College of Plant Protection NAU, for their beneficial suggestions and technical aid during the experiments. Our thanks also go to junior students, Ming Yang, Xiaoli Xiong and Zhu Tang, for their help in the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Monica Höfte.
Rights and permissions
About this article
Cite this article
Wu, Hs., Yang, Xn., Fan, Jq. et al. Suppression of Fusarium wilt of watermelon by a bio-organic fertilizer containing combinations of antagonistic microorganisms. BioControl 54, 287–300 (2009). https://doi.org/10.1007/s10526-008-9168-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-008-9168-7