Abstract
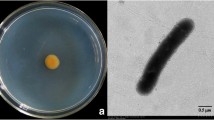
Pseudomonas aeruginosa GRC1 exhibited strong antagonistic activity against Sclerotinia sclerotiorum, in vitro and in vivo. Scanning electron microscopic (SEM) studies showed morphological abnormalities such as perforation, lysis and fragmentation of hyphae of S. sclerotiorum caused by P. aeruginosa GRC1. This strain produced extracellular chitinase enzyme, the role of which was clearly demonstrated through Tn5 mutagenesis. Bacterization of peanut seeds with GRC1 resulted in increased seed germination and reduced stem-rot of peanut in S. sclerotiorum-infested soil by 97%. Other vegetative and yield plant parameters such as nodules per plant, pods and grain yield per plant were enhanced with a statistical significance in comparison to control. Neomycin resistant (GRC neo+1 ) bacterium was a good root colonizer and frequently isolated from rhizosphere of peanut plants. These findings showed P. aeruginosa GRC1 as a potential biocontrol agent against S. sclerotiorum.
Similar content being viewed by others
Abbreviations
- SEM:
-
scanning electron microscopy
- HCN:
-
hydrocyanic acid
- CAS:
-
chrome azurol S
- IAA:
-
indole acetic acid
- TSM:
-
tryptic soy medium
- PDA:
-
potato dextrose agar
- CMM:
-
chitin minimal medium
- Km:
-
kanamycin
- neo:
-
neomycin
- CMC:
-
carboxymethylcellulose
- CFU:
-
colony forming unit
- DAS:
-
days after sowing
- ANOVA:
-
analysis of variants
References
Anas O. and Reeleder R.D. (1987). Recovery of fungi and arthropods from sclerotia of Sclerotinia sclerotiorum in québec muck soils. Phytopathology 77: 327–331
Arora N.K., Kang S.C., Maheshwari D.K. (2001) Isolation of siderophore producing strains of Rhizobium meliloti and their biocontrol potential againstMacrophomina phaseolina that causes charcoal rot of groundnut. Curr. Sci. 81: 673–677
Bakker A.W. and Schippers B. (1987). Microbial cyanides production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Microbiol. Biochem. 19: 451–457
Bakker P.A., Lamers H.M., Bakker A.W., Marugg J.D., Weisbeek P.J., Schippers B. (1986). The role of siderophore in potato tubers yield increase by Pseudomonas putida by a short relation potato. Neth. J. Plant Pathol. 92: 249–256
Bano N. and Musarrat J. (2002). Characterization of a new Pseudomonas aeruginosa NJ-15 as a potential biocontrol agent. Curr. Microbiol. 43: 182–186
Bhatia S., Dubey R.C., Maheshwari D.K. (2005). Enhancement of plant growth and suppression of collar rot of sunflower caused by Sclerotium rolferii through fluorescent pseudomonas. Ind. Phytopathol. 58: 17–24
Bhatia S., Bhatia S., Dubey R.C., Maheshwari D.K. (2003) Antagonistic effect of fluorescent pseudomonades against Macrophomina phaseolina that causes charcoal rot of groundnut. Ind. J. Exp. Biol. 41: 1442–1446
Boulnois G.J., Varly J.M., Sharpe G.S., Franklin F.C.H. (1985) Transposon donor plasmid, base on ColIBP9, for use in Pseudomonas putida and a variety of the Gram-negative bacteria. Mol. Gen. Genet. 200: 65–67
de Tempe J. (1963). The blotter method for seed health testing. Proc. Int. Seed Test Assoc. 28: 1933
Deshwal V.K., Dubey R.C., Maheshwari D.K. (2003). Isolation of plant growth-promoting Bradyrhizobium (Arachis) sp. with biocontrol potential against Macrophomina phaseolina causing charcoal rot of peanut. Curr. Sci. 84: 443–448
Dowling D.N., O’ Gara F.(1994). Metabolites of Pseudomonas involved in biocontrol of plant diseases. TIBTECH 12: 133–141
Dubey R.C., Dwivedi R.S. (1988) Effect of heavy metals on growth and survival of Macrophomina phaseolina (Tassi) Goid. Acta Bot. Ind. 16: 175–181
Dunne C., Crowley J.J., Moënne-Locooz Y., Dowling D.N., de Bruijn P.J., O’ Gara F. (1997). Biological control of Pythium ultimum by Stenotrophomonas maltopholia W81 is mediated by an extracellular proteolytic activity. Microbiology 143: 3921–3931
Fridlender, M., J. Inbar and I. Chet, 1993. Biological control of soil borne plant pathogens by \(\beta\)-1,3-glucanase producing Pseudomonas cepacia. Soil Biol. Biochem. 25(9): 1211–1221
Garbeva P., van Veen J.A., van Elas J.D. (2004). Assessment of the diversity and antagonism towards Rhizoctonia solani AG3 of Pseudomonas spp. in soil from different agricultural regimes. FEMS Microbiol. Ecol. 47: 51–64
Gomez K.A. and Gomez A.A. (1984). Statistical Procedures for Agricultural Research. A Wiley Interscience Publication, John Wiley and Sons, New York, USA
Gupta C.P., Sharma A., Dubey R.C., Maheshwari D.K. (1999). Pseudomonas aeruginosa as a strong antagonist of Macrophomina phaseolina and Fusarium oxysporum. Cytobios 99: 183–189
Gupta C.P., Dubey R.C., Maheshwari D.K. (2002). Plant growth enhancement and suppression of Macrophomina phaseolina causing charcoal rot of peanut by fluorescent Pseudomonas. Biol. Fertl. Soil 35: 295–301
Gupta C.P., Dubey R.C., Kang S.C., Maheshwari D.K. (2001). Antibiosis-mediated necrotrophic effect of Pseudomonas GRC2 against two fungal plant pathogens. Curr. Sci. 81: 91–94
Hofte M., Seong K.Y., Jurkevitch E., Verstraete W. (1991). Pyoverdin production by the plant growth beneficial Pseudomonas strain 7NSK2: Ecological significance in soil. Plant Soil 130: 249–257
Howel C.R. and Stipanovic C. (1980) Suppression of Pythium ultimum induced damping off of cotton seedlings by Pseudomonas fluorescens and its antibiotic pyoluteorin. Phytopathology 70: 712–715
Kloepper, J.W., R. Lifshitz and A. Novacky, 1988. Pseudomonas inoculation to benefit plant production. Animal Plant Sci. 60–64
Kumar Dileep B.S., Dubey H.C. (1992). Seed bacterization with a fluorescent Pseudomonas for enhanced plant growth, yield and disease control. Soil Biol. Biochem. 24: 539–542
Lee W.H. and Kobyashi K. (1980). Isolation and identification of antifungal Pseudomonas sp. from sugar beet roots and the use of antibiotic products. Korean J. Plant Pathol. 4: 264–270
Lim H.S., Kim S.D. (1994). The production and enzymatic properties of extracellular chitinase from Pseudomonas stutzeri YPL1 as a biocontrol agent. J. Microbiol. Biotechnol. 4: 134–140
Lim, H.S. and S.D. Kim, 1995. The role and characterization of \(\beta\)-1,3-glucanase in biocontrol of Fusarium solani by Pseudomonas stutzeri YLP-1. Curr. Microbiol. 33(4): 295–301
Palleroni N.J. (1984). Gram-negative aerobic rods and cocci: family I Pseudomonadaceae: genus I Pseudomonas. In: Krieg N.R., Holt J.G. (eds) Bergey’s Manual of Systematic Bacteriology, Vol. 1. William and Wilkins Co, Baltimore, MD, pp. 141–199
Purdy L.H. (1979). Sclerotinia sclerotiorum: history, diseases and symptomatology, host range, geographic distribution and impact. Phytopathology 69: 875–880
Ran L.X., Liu C.I., Wu G.J., van Loon L.C. and Bakker P.A.H.M. (2005). Suppression of bacterial wilt in Eucalyptus urophylla by fluorescens Pseudomonas spp. in China. Biol. Control 32: 111–120
Schipper B., Bakker A.W., Bakker P.A.H.M. (1987). Interaction of deleterious and beneficial rhizosphere microorganism and the effect on cropping practices. Ann. Rev. Phytopathol 25: 339–358
Schwyn B. and Neilands J.B. (1987). Universal assay for the detection and determination of siderophores. Anal. Biochem. 160: 47–56
Seong K.Y., Hofte M., Verstraete W. (1992). Acclimatization of plant growth promoting Pseudomonas strain 7NSK2 in soil. Effect on population dynamics and plant growth. Soil Biol. Biochem. 24: 75–759
Sivan A. and Chet I. (1989). Degradation of fungal cell walls by lytic enzymes of Trichoderma harzianum TK-1. J. Ferment. Bioeng. 78: 407–412
Skidmore A.M. and Dickinson C.H. (1976). Colony interaction and hyphal interference between Septoria nodorum and phylloplane fungi. Trans. Brit. Mycol. Soc. 66: 57–74
Steadman J.R. (1979). Control of plant diseases caused by Sclerotinia species. Symposium of Sclerotinia. Am. Phytopathol. Soc. 79: 904–907
Upadhyay R.S., Jayaswal R.K. (1992) Pseudomonas cepacia causes mycelial deformities and inhibition of condition in phytopathogenic fungi. Curr. Microbiol. 24: 181–187
Validov S., Mavrodi O., De La Fuente L., Boronin A., Weller D., Thomasho L., Mavrodi D. (2005). Antagonistic activity among 2,4-diacetyl phloroglucinol producing fluorescent Pseudomonas sp. FEMS Micribiol. Lett. 242: 249–256
Weller D.M. and Cook R.J. (1983). Suppression of take-all of wheat by seed treatments with fluorescent pseudomonads. Phytopathology 73: 463–469
Zhou T. and Boland G.J. (1999). Mycelial growth and production of oxalic acid by virulent and hypovirulent isolates of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 21: 93–99
Zhou T., Reeleder R.D. (1989). Application of Epicoccum purpurascens spores to control white mold of bean. Plant Dis. 73: 639-642
Acknowledgements
We thank Dr A. Ambani for the help with the Scanning Electron photo microscopy. This work was financially supported by TMOP - CSIR, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, C.P., Kumar, B., Dubey, R.C. et al. Chitinase-mediated destructive antagonistic potential of Pseudomonas aeruginosa GRC1 against Sclerotinia sclerotiorum causing stem rot of peanut. BioControl 51, 821–835 (2006). https://doi.org/10.1007/s10526-006-9000-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-006-9000-1