Abstract
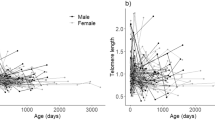
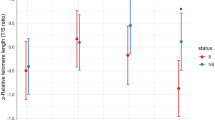
The questions about why and how senescence occurs in the wild are among the most pertinent ones in evolutionary ecology. Telomere length is a commonly used marker for aging, while other biomarkers of aging have received considerably less attention. Here we studied how another potent indicator of aging—skin pentosidine concentration—relates to age and blood telomere length in a long-lived seabird with well-documented reproductive senescence. We found no associations between telomere length, skin pentosidine and chronological age in male common gulls (Larus canus), aging from 2 to 30 years. However, the variance in telomere length was 4.6 times higher among the birds older than 13 years, which hints at relaxed selection on telomere length among the birds that have passed their prime age of reproduction. These results suggest that physiological and chronological ages may be largely uncoupled in our study system. Furthermore, our findings do not support a hypothesis about the presence of a common physiological factor (e.g., such as oxidative stress) that would cause covariation between two independent markers of aging.

Similar content being viewed by others
References
Asghar M, Hasselquist D, Bensch S (2011) Are chronic avian haemosporidian infections costly in wild birds? J Avian Biol 42:530–537
Asghar M, Bensch S, Tarka M, Hansson B, Hasselquist D (2014) Maternal and genetic factors determine early life telomere length. Proc R Soc Lond B 282:20142263
Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S (2015) Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347:436–438
Barrett ELB, Burke TA, Hammers M, Komdeur J, Richardson DS (2013) Telomere length and dynamics predict mortality in a wild longitudinal study. Mol Ecol 22:249–259
Bauch C, Becker PH, Verhulst S (2014) Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long-lived seabird. Mol Ecol 23:300–310. doi:10.1111/mec.12602
Brommer JE, Rattiste K (2008) “Hidden” reproductive conflict between mates in a wild bird population. Evolution 62:2326–2333
Brommer J, Rattiste K, Wilson A (2009) The rate of ageing in a long-lived bird is not heritable. Heredity 104:363–370
Chaney RC Jr, Blemings KP, Bonner J, Klandorf H (2003) Pentosidine as a measure of chronological age in wild birds. Auk 120:394–399. doi:10.2307/4090191
Cooey CK, Fallon JA, Avery ML, Anderson JT, Falkenstein EA, Klandorf H (2010) Refinement of biomarker pentosidine methodology for use on aging birds. Hum-Wild Interact 4:304–314
Criscuolo F, Bize P, Nasir L, Metcalfe NB, Foote CG et al (2009) Real-time quantitative PCR assay for measurement of avian telomeres. J Avian Biol 40:342–347
Fallon JA, Cochrane RL, Dorr B, Klandorf H (2006) Interspecies comparison of pentosidine accumulation and its correlation with age in birds. Auk 123:870–876
Haussmann MF, Marchetto NM (2010) Telomeres: linking stress and survival, ecology and evolution. Curr Zool 56:714–727
Haussmann MF, Mauck RA (2008) Telomeres and longevity: testing an evolutionary hypothesis. Mol Biol Evol 25:220–228
Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P (2012) Telomere length in early life predicts lifespan. P Natl Acad Sci USA 109:1743–1748
Holmes D, Martin K (2009) A bird’s-eye view of aging: what’s in it for ornithologists? Auk 126:1–23. doi:10.1525/auk.2009.1109
Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ (2008) Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med 44:235–246. doi:10.1016/j.freeradbiomed.2007.10.001
Ilmonen P, Kotrschal A, Penn DJ (2008) Telomere attrition due to infection. PLoS One 3:e2143. doi:10.1371/journal.pone.0002143
Iqbal M, Probert LL, Alhumadi NH, Klandorf H (1999) Protein glycosylation and advanced glycosylated endproducts (AGEs) accumulation: an avian solution? J Gerontol A 54:171–176
Jego M, Lemaitre JF, Bourgoin G, Capron G, Warnant C, Klein F, Gilot-Fromont E, Gaillard JM (2014) Haematological parameters do senesce in the wild: evidence from different populations of a long-lived mammal. J Evol Biol 27:2745–2752
Kilk K, Meitern R, Härmson O, Soomets U, Hõrak P (2014) Assessment of oxidative stress in serum by the d-ROMs test. Free Radic Res 48:883–889. doi:10.3109/10715762.2014.919390
Martin GM, Austad SN, Johnson TE (1996) Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet 13:25–34
Meitern R, Sild E, Kilk K, Porosk R, Hõrak P (2013) On the methodological limitations of detecting oxidative stress: effects of paraquat on measures of oxidative status in greenfinches. J Exp Biol 216:2713–2721. doi:10.1242/jeb.087528
Monaghan P (2014) Organismal stress, telomeres and life histories. J Exp Biol 217:57–66. doi:10.1242/jeb.090043
Monaghan P, Haussmann MF (2006) Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol 21:47–53. doi:10.1016/j.tree.2005.11.007
Nussey DH, Froy H, Lemaitre JF, Gaillard JM, Austad SN (2013) Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res Rev 12:214–225
Rattiste K (2004) Reproductive success in presenescent common gulls (Larus canus): the importance of the last year of life. Proc Roy Soc Lond B 271:2059–2064
Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S (2009) Telomere shortening and survival in free-living corvids. Proc Roy Soc Lond B 276:3157–3165
Sepp T, Sild E, Blount JD, Männiste M, Karu U, Hõrak P (2012) Individual consistency and covariation of measures of oxidative status in greenfinches. Phys Biochem Zool 85:299–307
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344. doi:10.1016/S0968-0004(02)02110-2
von Zglinicki T, Martin-Ruiz CM (2005) Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med 5:197–203. doi:10.2174/1566524053586545
Acknowledgments
We would like to thank Elizabeth Falkenstein for her technical help with measurement of skin pentosidine, and Ulvi Karu, Richard Meitern and Marju Männiste for their help in the field. The study was financed by the Estonian Ministry of Education and Research (Target-financing Project # 0180004s09, Institutional Research Grant # 2015), the European Union through the European Regional Development Fund (Centre of Excellence Frontiers in Biodiversity Research), Institutional Research Funding (Grants IUT21-1 and IUT34-8) by the Estonian Ministry of Education and Research, the West Virginia Agricultural and Forestry Experimental Station (H608), the Swedish Research Council (to DH), and partly by CAnMove (a Linneaus research excellence center at Lund University funded by the Swedish Research Council and Lund University). The study was conducted under license from the Committee of Animal Experiments at Estonian Ministry of Agriculture (decision # 5, issued on 20 April 2013).
Conflict of interest
We have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10522_2015_9564_MOESM1_ESM.doc
The datasets supporting this article have been uploaded as part of the supplementary material. Supplementary material 1 (DOC 195 kb)
Rights and permissions
About this article
Cite this article
Rattiste, K., Klandorf, H., Urvik, J. et al. Skin pentosidine and telomere length do not covary with age in a long-lived seabird. Biogerontology 16, 435–441 (2015). https://doi.org/10.1007/s10522-015-9564-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-015-9564-1