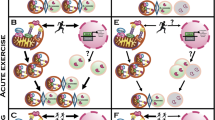
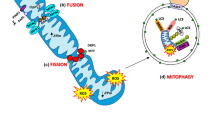
Abstract
Aging can be characterized as a time dependent decline of maximal functionality that affects tissues and organs of the whole body. Such is induced by the progressive loss of redundant components and leads to an increased susceptibility to disease and risk of death. Regarding the aging of skeletal muscle, it has been pointed out that mitochondria is a key factor behind the loss of redundancy and functionality, since this organelle has a major role in cellular homeostasis particularly at the level of the bioenergetic status. Decreased activities of the mitochondrial electron transport chain complexes and an increased release of reactive oxygen species from mitochondria are well documented with age; it is suggested that the mitochondrial loss of function results from the increased oxidative damage to proteins, lipids, and DNA of this organelle. However, it is important to be aware that the mitochondrial loss of function could also be a consequence, rather than a cause, of the cellular deterioration with age, which compromises mitochondrial biogenesis, mitochondrial protein turnover and autophagocytosis of damaged mitochondria. In this review several topics will be addressed regarding the age-related loss of skeletal muscle redundancy associated with mitochondrial dysfunction, emphasizing hypotheses for underlying mechanisms. In addition, we discuss some of the cellular mechanisms that can be pointed out as being responsible for the age-related mitochondrial dysfunction.



Similar content being viewed by others
References
Abuja PM, Albertini R (2001) Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin Chim Acta 306(1–2):1–17
Adhihetty P, Hood D (2003) Mechanisms of apoptosis in skeletal muscle. Basic Appl Myol 13(4):171–179
Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA (2003) Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol 88(1):99–107
Allen DL, Roy RR, Edgerton VR (1999) Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22(10):1350–1360
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70(2):200–214
Attardi G (2002) Role of mitochondrial DNA in human aging. Mitochondrion 2(1–2):27–37
Bakala H, Delaval E, Hamelin M, Bismuth J, Borot-Laloi C, Corman B, Friguet B (2003) Changes in rat liver mitochondria with aging. Lon protease-like reactivity and N(epsilon)-carboxymethyllysine accumulation in the matrix. Eur J Biochem 270(10):2295–2302
Barazzoni R, Short KR, Nair KS (2000) Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem 275(5):3343–3347
Barja G (1999) Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr 31(4):347–366
Barja G (2002) Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res Rev 1(3):397–411
Barja G (2004) Free radicals and aging. Trends Neurosci 27(10):595–600
Barja G, Herrero A (2000) Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J 14(2):312–318
Barja G, Cadenas S, Rojas C, Perez-Campo R, Lopez-Torres M (1994) Low mitochondrial free radical production per unit O2 consumption can explain the simultaneous presence of high longevity and high aerobic metabolic rate in birds. Free Radic Res 21(5):317–327
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78(2):547–581
Beckman KB, Ames BN (1999) Endogenous oxidative damage of mtDNA. Mutat Res 424(1–2):51–58
Bota DA, Davies KJ (2001) Protein degradation in mitochondria: implications for oxidative stress, aging and disease: a novel etiological classification of mitochondrial proteolytic disorders. Mitochondrion 1(1):33–49
Brierley EJ, Johnson MA, James OF, Turnbull DM (1997) Mitochondrial involvement in the ageing process. Facts and controversies. Mol Cell Biochem 174(1–2):325–328
Brookes PS (2005) Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med 38(1):12–23
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287(4):C817–C833
Bua EA, McKiernan SH, Wanagat J, McKenzie D, Aiken JM (2002) Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol 92(6):2617–2624
Bulteau AL, Szweda LI, Friguet B (2006) Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol 41(7):653–657
Butler RN, Austad SN, Barzilai N, Braun A, Helfand S, Larsen PL, McCormick AM, Perls TT, Shuldiner AR, Sprott RL, Warner HR (2003) Longevity genes: from primitive organisms to humans. J Gerontol A Biol Sci Med Sci 58(7):581–584
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29(3–4):222–230
Cao Z, Wanagat J, McKiernan SH, Aiken JM (2001) Mitochondrial DNA deletion mutations are concomitant with ragged red regions of individual, aged muscle fibers: analysis by laser-capture microdissection. Nucleic Acids Res 29(21):4502–4508
Carmeli E, Coleman R, Reznick AZ (2002) The biochemistry of aging muscle. Exp Gerontol 37(4):477–489
Coleman R, Silbermann M, Gershon D, Reznick AZ (1987) Giant mitochondria in the myocardium of aging and endurance-trained mice. Gerontology 33(1):34–39
Conley KE, Jubrias SA, Esselman PC (2000) Oxidative capacity and ageing in human muscle. J Physiol 526 Pt 1:203–210
Cortopassi G, Wang E (1995) Modelling the effects of age-related mtDNA mutation accumulation; complex I deficiency, superoxide and cell death. Biochim Biophys Acta 1271(1):171–176
Cortopassi GA, Wong A (1999) Mitochondria in organismal aging and degeneration. Biochim Biophys Acta 1410(2):183–193
Desai VG, Weindruch R, Hart RW, Feuers RJ (1996) Influences of age and dietary restriction on gastrocnemius electron transport system activities in mice. Arch Biochem Biophys 333(1):145–151
Dirks A, Leeuwenburgh C (2002) Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol 282(2):R519–R527
Dirks AJ, Leeuwenburgh C (2005) The role of apoptosis in age-related skeletal muscle atrophy. Sports Med 35(6):473–483
Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C (2006) Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev 5(2):179–195
Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H (2002) Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med 32(11):1102–1115
Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C (2003) Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol 284(2):R474–R480
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95
Duguez S, Feasson L, Denis C, Freyssenet D (2002) Mitochondrial biogenesis during skeletal muscle regeneration. Am J Physiol Endocrinol Metab 282(4):E802–E809
Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC (1999) Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci USA 96(9):4820–4805
Eto Y, Kang D, Hasegawa E, Takeshige K, Minakami S (1992) Succinate-dependent lipid peroxidation and its prevention by reduced ubiquinone in beef heart submitochondrial particles. Arch Biochem Biophys 295(1):101–106
Faist V, Koenig J, Hoeger H, Elmadfa I (1998) Mitochondrial oxygen consumption, lipid peroxidation and antioxidant enzyme systems in skeletal muscle of senile dystrophic mice. Pflugers Arch 437(1):168–171
Figueiredo PA, Mota MP, Appell HJ, Duarte J (2006) Ceasing of muscle function with aging: is it the consequence of intrinsic muscle degeneration or a secondary effect of neuronal impairments? Eur Rev Aging Phys Act 3(2):75–83
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408(6809):239–247
Floyd RA, West M, Hensley K (2001) Oxidative biochemical markers; clues to understanding aging in long-lived species. Exp Gerontol 36(4–6):619–640
Gardner PR, Raineri I, Epstein LB, White CW (1995) Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem 270(22):13399–13405
Gavrilov LA, Gavrilova NS (2004) The reliability-engineering approach to the problem of biological aging. Ann NY Acad Sci 1019:509–512
Gavrilov LA, Gavrilova NS (2006) Reliability theory of aging and longevity. In: Masoro EJ, Austad SN (eds) Handbook of the biology of aging. Academic Press, San Diego, pp 3–42
Gordon JW, Rungi AA, Inagaki H, Hood DA (2001) Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol 90(1):389–396
Grune T, Shringarpure R, Sitte N, Davies K (2001) Age-related changes in protein oxidation and proteolysis in mammalian cells. J Gerontol A Biol Sci Med Sci 56(11):B459–B457
Han D, Williams E, Cadenas E (2001) Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J 353(Pt 2):411–416
Han D, Antunes F, Canali R, Rettori D, Cadenas E (2003) Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278(8):5557–5563
Harman D (1998) Aging: phenomena and theories. Ann NY Acad Sci 854:1–7
Harman D (2001) Aging: overview. Ann NY Acad Sci 928:1–21
Herrero A, Barja G (1998) H2O2 production of heart mitochondria and aging rate are slower in canaries and parakeets than in mice: sites of free radical generation and mechanisms involved. Mech Ageing Dev 103(2):133–146
Hoch FL (1992) Cardiolipins and biomembrane function. Biochim Biophys Acta 1113(1):71–133
Hood DA (2001) Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90(3):1137–1157
Hood DA, Adhihetty PJ, Colavecchia M, Gordon JW, Irrcher I, Joseph AM, Lowe ST, Rungi AA (2003) Mitochondrial biogenesis and the role of the protein import pathway. Med Sci Sports Exerc 35(1):86–94
Ji LL, Fu R, Mitchell EW (1992) Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol 73(5):1854–1859
Kent-Braun JA, Ng AV (2000) Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol 89(3):1072–1078
Kowald A, Kirkwood TB (2000) Accumulation of defective mitochondria through delayed degradation of damaged organelles and its possible role in the ageing of post-mitotic and dividing cells. J Theor Biol 202(2):145–160
Kumaran S, Subathra M, Balu M, Panneerselvam C (2004) Age-associated decreased activities of mitochondrial electron transport chain complexes in heart and skeletal muscle: role of l-carnitine. Chem Biol Interact 148(1–2):11–18
Kwong LK, Sohal RS (2000) Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys 373(1):16–22
Larsen NB, Rasmussen M, Rasmussen LJ (2005) Nuclear and mitochondrial DNA repair: similar pathways? Mitochondrion 5(2):89–108
Lee CM, Lopez ME, Weindruch R, Aiken JM (1998) Association of age-related mitochondrial abnormalities with skeletal muscle fiber atrophy. Free Radic Biol Med 25(8):964–972
Leeuwenburgh C (2003) Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci 58(11):999–1001
Leeuwenburgh C, Heinecke JW (2001) Oxidative stress and antioxidants in exercise. Curr Med Chem 8(7):829–838
Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL (1994) Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol 267(2 Pt 2):R439–R445
Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL (1997) Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol 272(1 Pt 2):R363–R369
Lenaz G (1998) Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta 1366(1–2):53–67
Lenaz G, D’Aurelio M, Merlo Pich M, Genova ML, Ventura B, Bovina C, Formiggini G, Parenti Castelli G (2000) Mitochondrial bioenergetics in aging. Biochim Biophys Acta 1459(2–3):397–404
Linnane AW, Kios M, Vitetta L (2007) Healthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: the essential roles of superoxide anion and hydrogen peroxide. Biogerontology 8(5):445–467
Lyons CN, Mathieu-Costello O, Moyes CD (2006) Regulation of skeletal muscle mitochondrial content during aging. J Gerontol A Biol Sci Med Sci 61(1):3–13
Mailer K (1990) Superoxide radical as electron donor for oxidative phosphorylation of ADP. Biochem Biophys Res Commun 170(1):59–64
Mandavilli BS, Santos JH, Van Houten B (2002) Mitochondrial DNA repair and aging. Mutat Res 509(1–2):127–151
Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H (2006) Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev 127(3):298–306
Matsuo M, Kaneko T (2000) The chemistry of reactive oxygen species and related free radicals. In: Radák Z (ed) Free radicals in exercise and aging. Human Kinetics, Champaign, pp 1–33
McArdle A, Jackson MJ (2000) Exercise, oxidative stress and ageing. J Anat 197 Pt 4:539–541
Medvedev ZA (1990) An attempt at a rational classification of theories of ageing. Biol Rev Camb Philos Soc 65(3):375–398
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH (2006) Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61(6):534–540
Muller-Hocker J (1990) Cytochrome c oxidase deficient fibres in the limb muscle and diaphragm of man without muscular disease: an age-related alteration. J Neurol Sci 100(1–2):14–21
Nakahara H, Kanno T, Inai Y, Utsumi K, Hiramatsu M, Mori A, Packer L (1998) Mitochondrial dysfunction in the senescence accelerated mouse (SAM). Free Radic Biol Med 24(1):85–92
Nicholls DG (2002) Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol 34(11):1372–1378
Oh-ishi S, Heinecke JW, Ookawara T, Miyazaki H, Haga S, Radak Z, Kizaki T, Ohno H (2000) Role of lipid and lipoprotein oxidation. In: Radák Z (ed) Free radicals in exercise and aging. Human Kinetics, Champaign, pp 211–258
Okado-Matsumoto A, Fridovich I (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276(42):38388–38393
Ozawa T (1997) Genetic and functional changes in mitochondria associated with aging. Physiol Rev 77(2):425–464
Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E (1997) Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: role of cardiolipin. FEBS Lett 406(1–2):136–138
Paradies G, Petrosillo G, Pistolese M, Ruggiero FM (2002) Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 286(1):135–141
Passos JF, von Zglinicki T, Kirkwood TB (2007) Mitochondria and ageing: winning and losing in the numbers game. Bioessays 29(9):908–917
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI (2003) Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300(5622):1140–1142
Rasmussen UF, Rasmussen HN, Krustrup P, Quistorff B, Saltin B, Bangsbo J (2001) Aerobic metabolism of human quadriceps muscle: in vivo data parallel measurements on isolated mitochondria. Am J Physiol Endocrinol Metab 280(2):E301–E307
Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN (2003) Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria. Exp Gerontol 38(8):877–886
Rasmussen UF, Rasmussen HN (2000) Human skeletal muscle mitochondrial capacity. Acta Physiol Scand 168(4):473–480
Rattan SI (2006) Theories of biological aging: genes, proteins, and free radicals. Free Radic Res 40(12):1230–1238
Rooyackers OE, Adey DB, Ades PA, Nair KS (1996) Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 93(26):15364–15369
Sastre J, Pallardo FV, Vina J (2003) The role of mitochondrial oxidative stress in aging. Free Radic Biol Med 35(1):1–8
Scheffler I (2001) A century of mitochondrial research: achievements and perspectives Mitochondrion 1:3–31
Shigenaga MK, Hagen TM, Ames BN (1994) Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA 91(23):10771–10778
Short KR, Nair KS (2001) Does aging adversely affect muscle mitochondrial function? Exerc Sport Sci Rev 29(3):118–123
Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102(15):5618–5623
Sohal RS (2002) Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med 33(1):37–44
Sohal RS, Agarwal S, Dubey A, Orr WC (1993) Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci USA 90(15):7255–7259
Spiteller G (2001) Lipid peroxidation in aging and age-dependent diseases. Exp Gerontol 36(9):1425–1457
Stadtman ER (1992) Protein oxidation and aging. Science 257(5074):1220–1224
Stadtman ER (2002) Importance of individuality in oxidative stress and aging. Free Radic Biol Med 33(5):597–604
Stevnsner T, Thorslund T, de Souza-Pinto NC, Bohr VA (2002) Mitochondrial repair of 8-oxoguanine and changes with aging. Exp Gerontol 37(10–11):1189–1196
St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277(47):44784–44790
Takahashi M, Hood DA (1993) Chronic stimulation-induced changes in mitochondria and performance in rat skeletal muscle. J Appl Physiol 74(2):934–941
Terman A, Brunk UT (2004a) Aging as a catabolic malfunction. Int J Biochem Cell Biol 36(12):2365–2375
Terman A, Brunk UT (2004b) Myocyte aging and mitochondrial turnover. Exp Gerontol 39(5):701–705
Tonkonogi M, Sahlin K (2002) Physical exercise and mitochondrial function in human skeletal muscle. Exerc Sport Sci Rev 30(3):129–137
Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K (2003) Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch 446(2):261–269
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552(Pt 2):335–344
Turrens JF, Boveris A (1980) Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J 191(2):421–427
Valls V, Peiro C, Munizc P, Saez GT (2005) Age-related changes in antioxidant status and oxidative damage to lipids and dna in mitochondria of rat liver. Process Biochem 40:903–908
Van Remmen H, Hamilton ML, Richardson A (2003) Oxidative damage to DNA and aging. Exerc Sport Sci Rev 31(3):149–153
Vasilaki A, Mansouri A, Remmen H, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ (2006) Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5(2):109–117
Van Remmen H, Richardson A (2001) Oxidative damage to mitochondria and aging. Exp Gerontol 36(7):957–968
Ventura B, Genova ML, Bovina C, Formiggini G, Lenaz G (2002) Control of oxidative phosphorylation by Complex I in rat liver mitochondria: implications for aging. Biochim Biophys Acta 1553(3):249–260
Warner HR (2005) Longevity genes: from primitive organisms to humans. Mech Ageing Dev 126(2):235–242
Warner BB, Stuart L, Gebb S, Wispe JR (1996) Redox regulation of manganese superoxide dismutase. Am J Physiol 271(1 Pt 1):L150–L158
Waters DL, Brooks WM, Qualls CR, Baumgartner RN (2003) Skeletal muscle mitochondrial function and lean body mass in healthy exercising elderly. Mech Ageing Dev 124(3):301–309
Wei YH, Lee HC (2002) Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 227(9):671–682
Westermann B (2002) Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep 3(6):527–531
Yan LJ, Sohal RS (1998) Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci USA 95(22):12896–12901
Yan LJ, Levine RL, Sohal RS (1997) Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci USA 94(21):11168–11172
Yu BP (1994) Cellular defenses against damage from reactive oxygen species. Physiol Rev 74(1):139–162
Acknowledgments
The first author is co-financed by a grant of POCI 2010 and FSE. This work was supported by a grant of Fundação para a Ciência e Tecnologia (PTDC/10DES/70757/2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Figueiredo, P.A., Mota, M.P., Appell, H.J. et al. The role of mitochondria in aging of skeletal muscle. Biogerontology 9, 67–84 (2008). https://doi.org/10.1007/s10522-007-9121-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-007-9121-7