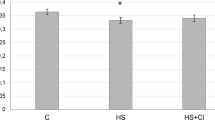
We studied the effect of histone deacetylase 1 (HDAC1) inhibition on titin content and expression of TTN gene in rat m. soleus after 3-day gravitational unloading. Male Wistar rats weighing 210±10 g were randomly divided into 3 groups: control, 3-day hindlimb suspension, and 3-day hindlimb suspension and injection of HDAC1 inhibitor CI-994 (1 mg/kg/day). In hindlimb-suspended rats, the muscle weight/animal body weight ratio was reduced by 13.8% (p<0.05) in comparison with the control, which attested to the development of atrophic changes in the soleus muscle. This was associated with a decrease in the content of NT-isoform of intact titin-1 by 28.6% (p˂0.05) and an increase in TTN gene expression by 1.81 times (p˂0.05) in the soleus muscle. Inhibition of HDAC1 by CI-994 during 3-day hindlimb suspension prevented the decrease in titin content and development of atrophy in rat soleus muscle. No significant differences in the TTN gene expression from the control were found. These results can be used when finding the ways of preventing or reducing the negative changes in the muscle caused by gravitational unloading.
Similar content being viewed by others
References
Vikhlyantsev IM, Podlubnaya ZA. New titin (connectin) isoforms and their functional role in mammalian striated muscles: facts and hypotheses. Uspekhi Biol. Khimii. 2012;52:239-280. Russian.
Beharry AW, Sandesara PB, Roberts BM, Ferreira LF, Senf SM, Judge AR. HDAC1 activates FoxO and is both sufficient and required for skeletal muscle atrophy. J. Cell Sci. 2014;127(Pt 7):1441-1453.
Cadar AG, Zhong L, Lin A, Valenzuela MO, Lim CC. Upstream open reading frame in 5’-untranslated region reduces titin mRNA translational efficiency. Biochem. Biophys. Res. Commun. 2014;453(1):185-191.
Di Lisa F, De Tullio R, Salamino F, Barbato R, Melloni E, Siliprandi N, Schiaffino S, Pontremoli S. Specific degradation of troponin T and I by mu-calpain and its modulation by substrate phosphorylation. Biochem. J. 1995;308(Pt 1):57-61.
Giger JM, Bodell PW, Zeng M, Baldwin KM, Haddad F. Rapid muscle atrophy response to unloading: pretranslational processes involving MHC and actin. J. Appl. Physiol. (1985). 2009;107(4):1204-1212.
Isaacs WB, Kim IS, Struve A, Fulton AB. Biosynthesis of titin in cultured skeletal muscle cells. J. Cell Biol. 1989; 109(5):2189-2195.
Koser F, Loescher C, Linke WA. Posttranslational modifications of titin from cardiac muscle: how, where, and what for? FEBS J. 2019;286(12):2240-2260.
Mohrhauser DA, Underwood KR, Weaver AD. In vitro degradation of bovine myofibrils is caused by μ-calpain, not caspase-3. J. Anim. Sci. 2011;89(3):798-808.
Moresi V, Carrer M, Grueter CE, Rifki OF, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylases 1 and 2 regulate autophagy flux and skeletal muscle homeostasis in mice. Proc. Natl Acad. Sci. USA. 2012;109(5):1649-1654.
Murphy RM, Snow RJ, Lamb GD. mu-Calpain and calpain-3 are not autolyzed with exhaustive exercise in humans. Am. J. Physiol. Cell Physiol. 2006;290(1):C116-C122.
Toursel T, Stevens L, Granzier H, Mounier Y. Passive tension of rat skeletal soleus muscle fibers: effects of unloading conditions. J. Appl. Physiol. (1985). 2002;92(4):1465-1472.
Udaka J, Ohmori S, Terui T, Ohtsuki I, Ishiwata S, Kurihara S, Fukuda N. Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. J. Gen. Physiol. 2008;131(1):33-41.
Ulanova A, Gritsyna Y, Salmov N, Lomonosova Y, Belova S, Nemirovskaya T, Shenkman B, Vikhlyantsev I. Effect of L-arginine on titin expression in rat soleus muscle after hindlimb unloading. Front. Physiol. 2019;10. ID 1221. https://doi.org/10.3389/fphys.2019.01221
Ulanova A, Gritsyna Y, Vikhlyantsev I, Salmov N, Bobylev A, Abdusalamova Z, Rogachevsky V, Shenkman B, Podlubnaya Z. Isoform composition and gene expression of thick and thin filament proteins in striated muscles of mice after 30-day space flight. Biomed. Res. Int. 2015;2015. ID 104735. https://doi.org/10.1155/2015/104735
Walsh ME, Van Remmen H. Emerging roles for histone deacetylases in age-related muscle atrophy. Nutr. Healthy Aging. 2016;4(1):17-30.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 169, No. 4, pp. 431-438, April, 2020
Rights and permissions
About this article
Cite this article
Ulanova, A.D., Gritsyna, Y.V., Bobylev, A.G. et al. Inhibition of Histone Deacetylase 1 Prevents the Decrease in Titin (Connectin) Content and Development of Atrophy in Rat m. soleus after 3-Day Hindlimb Unloading. Bull Exp Biol Med 169, 450–457 (2020). https://doi.org/10.1007/s10517-020-04907-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-020-04907-5