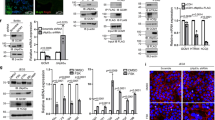
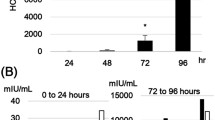
Hypoxia of trophoblast cells is an important regulator of normal development of the placenta. However, some pathological states associated with hypoxia, e.g. preeclampsia, impair the functions of placental cells. Oxyquinoline derivative inhibits HIF-prolyl hydroxylase by stabilizing HIF-1 transcription complex, thus modeling cell response to hypoxia. In human choriocarcinoma cells BeWo b30 (trophoblast model), oxyquinoline increased the expression of a core hypoxia response genes along with up-regulation of NOS3, PDK1, and BNIP3 genes and down-regulation of the PPARGC1B gene. These changes in the expression profile attest to activation of the metabolic cell reprogramming mechanisms aimed at reducing oxygen consumption by enabling the switch from aerobic to anaerobic glucose metabolism and the respective decrease in number of mitochondria. The possibility of practical use of the therapeutic properties of oxyquinoline derivatives is discussed.
Similar content being viewed by others
References
Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37(14):4587-4602.
Fomicheva KA, Osip’yants AI, Knyazev EN, Samatov TR, Shkurnikov MY. Detection of potential Metastatic prostate cancer circulating biomarkers by comparison of miRNA profiles in DU145 cells and culture medium. Bull. Exp. Biol. Med. 2017;162(6):792-796.
Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int. J. Dev. Biol. 2010;54(2-3):409-419.
Knyazev EN, Fomicheva KA, Mikhailenko DS, Nyushko KM, Samatov TR, Alekseev BY, Shkurnikov MY. Plasma levels of hsa-miR-619-5p and hsa-miR-1184 differ in prostatic benign hyperplasia and cancer. Bull. Exp. Biol. Med. 2016;161(1):108-111.
Knyazev EN, Mal’tseva DV, Zacharyants AA, Zakharova GS, Zhidkova OV, Poloznikov AA. TNFα-induced expression of transport protein genes in HUVEC cells is associated with enhanced expression of transcription factor genes RELB and NFKB2 of the non-canonical NF-κB Pathway. Bull. Exp. Biol. Med. 2018;164(6):757-761.
Knyazev EN, Mal’tseva DV, Zakharyants AA, Zakharova GS, Zhidkova OV, Poloznikov AA. Expression of microRNA genes MIR221, MIR222, and MIR181B1 increases during induction of inflammation in the endothelial barrier model. Bull. Exp. Biol. Med. 2018;164(6):749-752.
Knyazev EN, Nyushko KM, Alekseev BY, Samatov TR, Shkurnikov MY. Suppression of ITGB4 gene expression in PC-3 cells with short interfering RNA induces changes in the expression of β-integrins associated with RGD-receptors. Bull. Exp. Biol. Med. 2015;159(4):541-545.
Macklin PS, McAuliffe J, Pugh CW, Yamamoto A. Hypoxia and HIF pathway in cancer and the placenta. Placenta. 2017;56):8-13.
Osipyants AI, Smirnova NA, Khristichenko AY, Hushpulian DM, Nikulin SV, Chubar TA, Zakhariants AA, Tishkov VI, Gazaryan IG, Poloznikov AA. Enzyme-substrate reporters for evaluation of substrate specificity of HIF prolyl hydroxylase isoforms. Biochemistry (Mosc). 2017;82(10):1207-1214.
Poloznikov AA, Zakhariants AA, Nikulin SV, Smirnova NA, Hushpulian DM, Gaisina IN, Tonevitsky AG, Tishkov VI, Gazaryan IG. Structure-activity relationship for branched oxyquinoline HIF activators: Effect of modifications to phenylacetamide “tail”. Biochimie. 2017;133:74-79.
Rudimov EG, Knjazev EN, Khaustova NA, Grigorieva OV, Buravkova LB. Transcriptomic changes in human umbilical cord blood endothelial cells under simulated microgravity. Dokl. Biochem. Biophys. 2017;472(1):1-4.
Shkurnikov MY, Knyazev EN, Wicklein D, Schumacher U, Samatov TR, Tonevitskii AG. Role of L1CAM in the Regulation of the canonical Wnt pathway and class I MAGE genes. Bull. Exp. Biol. Med. 2016;160(6):807-810.
Skurnikov MY, Makarova YA, Knyazev EN, Fomicheva KA, Nyushko KM, Saribekyan EK, Alekseev BY, Kaprin AD. Profile of microRNA in blood plasma of healthy humans. Bull. Exp. Biol. Med. 2016;160(5):632-634.
Smirnova NA, Kaidery NA, Hushpulian DM, Rakhman II, Poloznikov AA, Tishkov VI, Karuppagounder SS, Gaisina IN, Pekcec A, Leyen KV, Kazakov SV, Yang L, Thomas B, Ratan RR, Gazaryan IG. Bioactive flavonoids and catechols as Hif1 and Nrf2 protein stabilizers — implications for Parkinson’s disease. Aging Dis. 2016;7(6):745-762.
Thompson CB. Into thin air: How we sense and respond to hypoxia. Cell. 2016;167(1):9-11.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 165, No. 9, pp. 290-295, September, 2018
Rights and permissions
About this article
Cite this article
Knyazev, E.N., Zakharova, G.S., Astakhova, L.A. et al. Metabolic Reprogramming of Trophoblast Cells in Response to Hypoxia. Bull Exp Biol Med 166, 321–325 (2019). https://doi.org/10.1007/s10517-019-04342-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-019-04342-1