Abstract
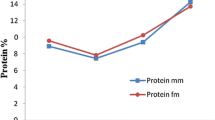
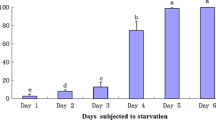
Lipid composition of the mantle and digestive gland of Octopus vulgaris that were not fed for 27 days were determined. Every 3 days, three octopuses were killed and samples of the mantle and the digestive gland (DG) were taken, in order to determine total lipids as well as lipid classes and fatty acids. Composition in total lipids (TL) for the mantle was similar until day 21, then decreased and remained similar until the end of the experiment. Composition in total lipids for the DG decreased significantly after 3 days, then remained similar until day 21, and then decreased until the end of the experiment. As for the lipid classes, in the DG the main components were triglycerides and sterol esters. Sterol esters suffered strong reductions after 10 days of starvation, while triglycerides remained similar until day 21 and then decreased until the end of the experiment. Cholesterol decreased gradually throughout the experimental period. For polar lipids, phosphatidylcholine and phosphatidylethanolamine increased during the first 3 days and then decreased throughout the experiment. In the mantle, the only neutral classes that decrease were triacylglycerols and sterol esters, while no polar lipid classes decreased in this organ. It was noticeable the decrease in almost all fatty acids in the DG after 3 days of starvation, while in the mantle there were no differences in fatty acid concentrations during the experiment.



Similar content being viewed by others
References
Aguado-Giménez F, García-García B (2003) Growth and food intake models in Octopus vulgaris Cuvier (1797): influence of body weight, temperature, sex and diet. Aquacult Int 10:361–377
Almansa E, Sánchez JJ, Cozzi S, Rodríguez C, Díaz M (2003) Temperature–activity relationship for the intestinal Na+-K+-ATPase of Sparus aurata. A role for the phospholipid microenvironment? J Comp Physiol B 173:231–237
Almansa E, Domingues P, Sykes A, Tejera N, Lorenzo A, Andrade J (2006) The effects of feeding with shrimp or fish fry on growth and mantle lipid composition of juvenile and adult cuttlefish (Sepia officinalis). Aquaculture 256:403–413
Ballantyne JS, Hochachka PW, Mommsen TP (1981) Studies on the metabolism of the migratory squid, Loligo opalescens: enzymes of tissues and heart mitochondria. Mar Biol Lett 2:75–85
Boucaud-Camou E, Boucher-Rodoni R (1983) Feeding and digestion in cephalopods. Mollusca Physiol 5(2):149–187
Boucaud-Camou E, Yim M (1980) Fine structure and function of the digestive cell of Sepia officinalis (Mollusca: Cephalopoda). J Zool 191:89–105
Boucaud-Camou E, Yim M, Tresgot A (1985) Feeding and digestion of young Sepia officinalis L. during post-hatching development. Vie Milieu 35:263–266
Boucher-Rodoni R (1989) Consommation d’oxygéne et excrétion ammoniacale de Nautilus macrophalus. C r hebd Séanc Acad Sci Paris 309:173–179
Boucher-Rodoni R, Mangold K (1988) Comparative aspects of ammonia excretion in cephalopods. Malacologia 29:145–151
Boucher-Rodoni R, Boucaud-Camou E, Mangold K (1987) Feeding and digestion. In: Boyle P (ed) Cephalopod life cycles. Academic Press, London, pp 85–108
Castro BG (1990) Can Sepia officinalis L. be reared on artificial food? Mar Behav Physiol 19:35–38
Castro BG, Lee PG (1994) The effects of semi-purified diets on growth and condition of Sepia officinalis L (Mollusca: Cephalopoda). Comp Biochem Physiol 109:1007–1016
Castro BG, Garrido JL, Sotelo CG (1992) Changes in composition of digestive gland and mantle muscle of the cuttlefish Sepia officinalis during starvation. Mar Biol 114:11–20
Castro B, DiMarco FP, DeRusha RH, Lee PG (1993) The effects of surimi and pelleted diets on the laboratory survival, growth, and feeding rate of the cuttlefish Sepia officinalis L. J Exp Mar Biol Ecol 170:241–252
Cerezo-Valverde J, Hernández M, Aguado-Giménez F, García-García B (2008) Growth, feed efficiency and condition of common octopus (Octopus vulgaris) fed on two formulated moist diets. Aquaculture 275:266–273
Christie WW (1982) Lipids analysis, 2nd edn. Pergamon Press, Oxford
Clarke A, Rodhouse PG, Holmes LJ, Pascoe PL (1989) Growth rate and nucleic acid ratio in cultured cuttlefish Sepia officinalis (Mollusca: Cephalopoda). J Exp Mar Biol Ecol 133:229–240
DeRusha RH, Forsythe JW, DiMarco FP, Hanlon RT (1989) Alternative diets for maintaining and rearing cephalopods in captivity. Lab Ani Sci 39(4):306–312
Domingues P (1999) Development of alternative diets for the mass culture of the European cuttlefish Sepia officinalis. PhD thesis, University of the Algarve, Portugal, 95 p
Domingues P, Poirier R, Dickel L, Almansa E, Sykes A, Andrade JP (2003) Effects of culture density and live prey on growth and survival of juvenile cuttlefish, Sepia officinalis. Aquacult Int 11:225–242
Domingues PM, DiMarco FP, Andrade JP, Lee PG (2005) The effects of diets with amino acid supplementation on the survival, growth and body composition of the cuttlefish Sepia officinalis. Aquacult Int 13:423–440
Domingues P, Bettencourt V, Guerra A (2006) Growth of Sepia officinalis in captivity and in nature. Vie Millieu 56:109–120
Domingues P, López N, Muñoz J, Maldonado T, Gaxiola G, Rosas C (2007) Effects of an artificial diet on growth and survival of the Yucatan octopus, Octopus maya. Aquac Nutr 13:273–280
Domingues P, García S, Hachero-Cruzado I, López N, Rosas C (2009) The use of alternative prey (crayfish, Procambarus clarkii, and hake, Merlucius gayi) to culture Octopus vulgaris (Cuvier, 1797). Aquacult Int. doi:10.1007/s10499-009-9259-1
Dunstan GA, Baillie HJ, Barrett SM, Volkman JK (1996) Effect of diet on the lipid composition of wild and cultured abalone. Aquacult Abalone Cult 140:115–127
Durazo-Beltrán E, D’Abramo LR, Toro-Vazquez JF, Vasquez-Peláez C, Viana MT (2003) Effect of triacylglycerols in formulated diets on growth and fatty acid composition in tissue of green abalone (Haliotis fulgens). Aquaculture 224:257–270
FAO (2008) The state of the world fisheries, aquaculture 2008. SOFIA, Rome, Italy 218 p
Ferreira A, Marquez L, Andrade J, Lorenzo A, Domingues P (2009) The use of alternative diets to culture juvenile cuttlefish, Sepia officinalis: effects on growth and lipid composition. Aquac Nutr. doi:10.1111/j.1365-2095.2009.00661.x
Fluckiger M, Jackson GD, Nichols P, Virtue P, Daw A, Wotherspoon S (2008) An experimental study of the effect of diet on the fatty acid profiles of the European Cuttlefish (Sepia officinalis). Mar Biol 154:363–372
Fowler J, Cohen L, Jarvis P (2002) Practical statistics for field biology, 2nd edn. Wiley, West Sussex, England 259 pp
García S, Domingues P, Navarro JC, Hachero-Cruzado I, Garrido D, Rosas C (2009) Growth, partial energy balance, mantle and digestive gland lipid composition of Octopus vulgaris (Cuvier, 1797) fed with to artificial diets. Aquac Nutr. doi:10.1111/j.1365-2095.2009.00746.x
Heras H, Pollero RJ (1989) Blood lipids of the small octopus Octopus tehuelchus (Mollusca: Cephalopoda) at different stages of sexual maturation. Comp Biochem Physiol 92 A:571–575
Heras H, Pollero RJ (1990) Occurrence of plasma lipoproteins in octopods. Partial characterization and interorgan transport of lipids. J Exp Mar Biol Ecol 140:29–38
Horrocks LA, Farooqui AA (2004) Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fat Acids 70:361–372
Horwitz W (1980) Methods of analysis, 13th edn. Association of Official Analytical Chemists, Washington DC
Iglesias J, Sánchez FJ, Otero JJ (1997) Primeras experiencias sobre el cultivo integral del pulpo (Octopus vulgaris Cuvier) en el IEO. In: Costa J, Abellán E, García B, Ortega A, Zamora S (eds), Actas del VI C Nacional de Acuicultura. Cartagena 1997. ISBN: 84-491-0323-1, 221–226 pp
Iglesias J, Fuentes L, Sánchez J, Otero JJ, Moxica C, Lago MJ (2006) First feeding of Octopus vulgaris Cuvier, 1797 paralarvae using Artemia: effect of prey size, prey density and feeding frequency. Aquaculture 261:817–822
Iglesias J, Sánchez FJ, Bersano JGF, Carrasco JF, Dhont J, Fuentes L, Linares F, Muñoz JL, Okumura S, Roo J, van der Meeren T, Vidal EAG, Villanueva R (2007) Rearing of Octopus vulgaris paralarvae: present status, bottlenecks and trends. Aquaculture 266:1–15
Itami K, Izawa Y, Maeda S, Nakai K (1963) Notes on the laboratory culture of the octopus larvae. Bull Jap Soc Sci Fish 29:514–520
Lee PG (1994) Nutrition of cephalopods: fuelling the system. Mar Freshw Behav Physiol 25:35–51
Lee PG, Forsythe JW, DiMarco FP, DeRusha R, Hanlon RT (1991) Initial palatability and growth trials on pelleted diets for cephalopods. Bull Mar Sci 49:362–372
Mangold K (1983) Food, feeding and growth in cephalopods. Mem Natl Mus Vic 44:81–93
Miliou H, Fintikaki M, Tzitzinakis M, Kountouris T, Verriopoulos G (2006) Fatty acid composition of the octopus, Octopus vulgaris, in relation to rearing temperature and body weight. Aquaculture 256:311–322
Moltschaniwskyj NA, Jackson GD (2000) Growth and tissue composition as a function of feeding history in juvenile cephalopods. J Exp Mar Biol Ecol 253:229–241
Moltschaniwskyj NA, Johnston D (2006) Evidence that lipid can be digested by the dumpling squid Euprymna tasmanica, but is not stored in the digestive gland. Mar Biol 149:565–572
Mommsen TP, Hochachka PW (1981) Respiratory and enzymatic properties of squid heart mitochondria. Eur J Biochem 120:345–350
Moreno JEA, Moreno VJ, Ricci L, Roldán M, Gerpe M (1998) Variations in the biochemical composition of the squid Illex argentinus from the South Atlantic Ocean. Comp Biochem Physiol 119B:631–637
Navarro JC, Villanueva R (2000) Lipid and fatty acid composition of early stage of cephalopods: an approach to the lipid requirements. Aquaculture 183:161–177
Navarro JC, Villanueva R (2003) The fatty acid composition of Octopus vulgaris paralarvae reared with live and inert food: deviation from their natural profile. Aquaculture 219:613–631
O’Dor RK, Boucher-Rodoni R, Wells MJ, Wells J (1984) Nutrient absorption, storage and remobilization in Octopus vulgaris. Mar Behav Pysiol 11:239–258
Olsen RE, Henderson RJ (1989) The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry. J Exp Mar Biol Ecol 129(2):189–197
Perrin A, Le Bihan E, Koueta N (2004) Experimental study of enriched frozen diet on digestive enzymes and growth of juvenile cuttlefish Sepia officinalis L. (Mollusca Cephalopoda). J Exp Mar Biol Ecol 311:267–285
Quintana D, Domingues P, García S (2008) Effects of two artificial wet diets agglutinated with gelatin on feed and growth performance of common octopus (Octopus vulgaris) sub-adults. Aquaculture 280:161–164
Rosa R, Pereira J, Nunes ML (2005) Biochemical composition of cephalopods with different life strategies, with special reference to giant squid, Architeuthis sp. Mar Biol 146:739–751
Rosas C, Cuzon G, Pascual C, Gaxiola G, López N, Maldonado T, Domingues P (2007) Energy balance of Octopus maya fed crab and artificial diet. Mar Biol 152:371–378
Sargent JR, Bell JG, Henderson RJ, Tocher DR (1995) Origins and functions of n-3 polyunsaturated fatty acids in marine organisms. In: Ceve G, Paltauf F (eds) Phospholipids: characterization, metabolism and novel biochemical applications. American Oil Chemical Society Press, Champaign, IL, pp 248–259
Segawa S, Hanlon TR (1988) Oxygen consumption and ammonia excretion rates in Octopus maya, Loligo forbesi and lolliguncula brevis (Mollusca: Cephalopoda). Mar Behav Physiol 13:389–400
Semmens JM, Moltschaniwskyj NA, Alexander CG (1995) Effect of feeding on the digestive gland of the tropical sepioid Idiosepius pygmaeus. J Mar Biol Ass UK 75:885–897
Sinanoglou VJ, Miniadis-Meimaroglou S (1998) Fatty acid of neutral and polar lipids of (edible) Mediterranean cephalopods. Food Res Int 31:467–473
Sinanoglou VJ, Miniadis-Meimaroglou S (2000) Phospholipids in Mediterranean cephalopods. J Biosci 55:245–255
Tait RW (1986) Aspects physiologiques de la sénescence post reproductive chez Octopus vulgaris. Ph D Thése L’Université Paris VI
Tocher DR (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci 11:107–184
Uki N, Sugiura M, Watanabe T (1986) Requirement of essential fatty acids in the abalone Haliotis discus hannai. Bull Jpn Sot Sci Fish 52:1013–1023 (in Japanese, with English abstract)
Villanueva R, Koueta N, Riba J, Boucaud-Camou E (2002) Growth and proteolytic activity of Octopus vulgaris paralarvae with different food rations during first feeding, using Artemia nauplii and compound diets. Aquaculture 205:269–286
Young RE, Harman RF (1988) “Larva”, “paralarvae” and “subadult” in cephalopod terminology. Malacologia 29:201–207
Zar JH (1999) In: Ryu T (ed) Biostatistical analysis, 4th edition. Prentice-Hall Inc, Upper Saddle River, NJ, 663 p
Zlatanos S, Laskaridis K, Feist C, Sagredos A (2006) Proximate composition, fatty acid analysis and protein digestibility-corrected amino acid score of three Mediterranean cephalopods. Mol Nutr Food Res 50:967–970
Acknowledgments
The authors wish to thank the “Plan Nacional de pulpo”—JACUMAR—Project “Engorde de pulpo, Octopus vulgaris.” 2007/2009, for the funding for this research. Sandra García-Garrido wishes to thank the “Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria” (INIA) for the Pre-doctoral grant no 47 (BOE no 308 26/12/2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Garrido, S., Hachero-Cruzado, I., Garrido, D. et al. Lipid composition of the mantle and digestive gland of Octopus vulgaris juveniles (Cuvier, 1797) exposed to prolonged starvation. Aquacult Int 18, 1223–1241 (2010). https://doi.org/10.1007/s10499-010-9335-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-010-9335-6