Abstract
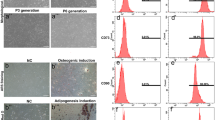
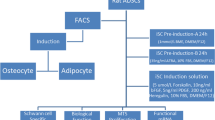
Schwann cells (SCs) have important roles in supporting and repairing peripheral neurons, and thus have great potential for nerve injury treatment. Adipose tissue-derived stem cells (ADSCs) can be reliably induced to differentiate into SCs. However, the underlying molecular mechanisms are unclear. We explored the roles of MEG3/let-7a-5p/RBPJ axis in the differentiation into SCs from ADSCs. Primary ADSCs were induced to differentiate into SCs by appropriate reagents. ELISA, immunostaining, Western blotting, and qRT-PCR were employed to examine levels of SC-markers such as S100, GFAP, SOX10, p75NTR, GAP43, MPZ, β-NGF, BDNF, and NCAM and let-7 family, MEG3, RBPJ, and Notch signaling related proteins. Dual luciferase assay and RNA immunoprecipitation were performed to validate interactions of let-7a-5p/RBPJ mRNA and MEG3/let-7a-5p. Cultured ADSCs could be induced to differentiate into functional SCs. Let-7a-5p and let-7d-5p were elevated during the differentiation while MEG3 and RBPJ/Notch-signaling were suppressed. Let-7a-5p mimics promoted ADSC differentiation into SCs and up-regulated the levels of SC-related markers including S100, GFAP, SOX10, p75NTR, GAP43, MPZ, β-NGF, and NCAM, while RBPJ or MEG3 overexpression retarded the differentiation and reduced those levels. Let-7a-5p directly targeted RBPJ and MEG3 disinhibited Notch-RBPJ signaling via sponging let-7a-5p. RBPJ overexpression reversed the acceleration of let-7a-5p mimics on SC differentiation while let-7a-5p mimics blocked MEG3-mediated suppression on SC differentiation. Let-7a-5p sponged by MEG3 promotes differentiation of ADSCs into SCs via suppressing Notch signaling by targeting RBPJ. These findings shed light on mechanisms underlying the differentiation of ADSCs to SCs and provide avenues to accelerate the process.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
Abbreviations
- SC:
-
Schwann cell
- NSC:
-
Neural stem cell
- ADSC:
-
Adipose tissue-derived stem cell
- NICD:
-
Notch intracellular domain
- RBPJ:
-
Recombination signal-binding protein for immunoglobulin κ J region
- BMSC:
-
Bone marrow mesenchymal stem cell
- ncRNA:
-
Non-coding RNAs
- miRNA:
-
Micro RNA
- lncRNA:
-
Long non-coding RNA
- ceRNA:
-
Competing endogenous RNA
- MEG3:
-
Maternally expressed gene 3
- DMEM:
-
Dulbecco's Modified Eagle Medium
- FBS:
-
Fetal bovine serum
- PDGF:
-
Platelet-derived growth factor
- FFGF:
-
Fibroblast Growth Factor
- β-NGF:
-
β-Nerve growth factor
- BDNF:
-
Brain-derived neurotrophic factor
- IL-6:
-
Interleukin 6
- NCAM:
-
Neural cell adhesion molecule
- GFAP:
-
Glial fibrillary acidic protein
- HES1/5:
-
Hairy and enhancer of split 1/5
References
Jessen KR, Mirsky R, Lloyd AC (2015) Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol 7:a020487
Jessen KR, Mirsky R (2019) The success and failure of the schwann cell response to nerve injury. Front Cell Neurosci 13:33
Jessen KR, Mirsky R (2016) The repair Schwann cell and its function in regenerating nerves. J Physiol 594:3521–3531
Kanno H, Pearse DD, Ozawa H, Itoi E, Bunge MB (2015) Schwann cell transplantation for spinal cord injury repair: its significant therapeutic potential and prospectus. Rev Neurosci 26:121–128
Si Z, Wang X, Sun C et al (2019) Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. Biomed Pharmacother 114:108765
Miana VV, Gonzalez EAP (2018) Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience 12:822
Chu DT, Nguyen Thi Phuong T, Tien NLB et al (2019) Adipose tissue stem cells for therapy: an update on the progress of isolation, culture, storage, and clinical application. J Clin Med 8:917
Sun X, Zhu Y, Yin HY et al (2018) Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: potential advantage of cellular transient memory function. Stem Cell Res Ther 9:133
Xie S, Lu F, Han J et al (2017) Efficient generation of functional Schwann cells from adipose-derived stem cells in defined conditions. Cell Cycle 16:841–851
Zong D, Ouyang R, Li J, Chen Y, Chen P (2016) Notch signaling in lung diseases: focus on Notch1 and Notch3. Ther Adv Respir Dis 10:468–484
Jiang J, Xiao K, Chen P (2017) NOTCH signaling in lung diseases. Exp Lung Res 43:217–228
Bray SJ (2016) Notch signalling in context. Nat Rev Mol Cell Biol 17:722–735
Henrique D, Schweisguth F (2019) Mechanisms of Notch signaling: a simple logic deployed in time and space. Development 146:1185–1196
Siebel C, Lendahl U (2017) Notch signaling in development, tissue homeostasis, and disease. Physiol Rev 97:1235–1294
Yin J, Huang F, Yi Y, Yin L, Peng D (2016) EGCG attenuates atherosclerosis through the Jagged-1/Notch pathway. Int J Mol Med 37:398–406
He Y, Zou L (2019) Notch-1 inhibition reduces proliferation and promotes osteogenic differentiation of bone marrow mesenchymal stem cells. Exp Ther Med 18:1884–1890
Osathanon T, Subbalekha K, Sastravaha P, Pavasant P (2012) Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol Int 36:1161–1170
Liu Z, Wang Y, Shu S, Cai J, Tang C, Dong Z (2019) Non-coding RNAs in kidney injury and repair. Am J Physiol Cell Physiol 317:C177–C188
Palazzo AF, Lee ES (2015) Non-coding RNA: what is functional and what is junk? Front Genet 6:2
Su JL, Chen PS, Johansson G, Kuo ML (2012) Function and regulation of let-7 family microRNAs. Microrna 1:34–39
Yao RW, Wang Y, Chen LL (2019) Cellular functions of long noncoding RNAs. Nat Cell Biol 21:542–551
Shi Y (2020) MEG3 regulates apoptosis of adiposederived stem cells. Mol Med Rep 21:2435–2442
Zhuang W, Ge X, Yang S et al (2015) Upregulation of lncRNA MEG3 promotes osteogenic differentiation of mesenchymal stem cells from multiple myeloma patients by targeting BMP4 transcription. Stem Cells 33:1985–1997
Xu Y, Liu Z, Liu L et al (2008) Neurospheres from rat adipose-derived stem cells could be induced into functional Schwann cell-like cells in vitro. BMC Neurosci 9:21
Liew LJ, Ong HT, Dilley RJ (2017) Isolation and culture of adipose-derived stromal cells from subcutaneous fat. Methods Mol Biol 1627:193–203
Dechant G, Barde YA (2002) The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci 5:1131–1136
Jessen KR, Mirsky R (2005) The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6:671–682
Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M (1998) Sox10, a novel transcriptional modulator in glial cells. J Neurosci 18:237–250
Liu Z, Jin YQ, Chen L et al (2015) Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS ONE 10:e0123278
Lee H, Han S, Kwon CS, Lee D (2016) Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell 7:100–113
Sun X, Liu J, Xu C, Tang SC, Ren H (2016) The insights of Let-7 miRNAs in oncogenesis and stem cell potency. J Cell Mol Med 20:1779–1788
Bussing I, Slack FJ, Grosshans H (2008) let-7 microRNAs in development, stem cells and cancer. Trends Mol Med 14:400–409
Li S, Wang X, Gu Y et al (2015) Let-7 microRNAs regenerate peripheral nerve regeneration by targeting nerve growth factor. Mol Ther 23:423–433
Gokbuget D, Pereira JA, Bachofner S et al (2015) The Lin28/let-7 axis is critical for myelination in the peripheral nervous system. Nat Commun 6:8584
Sohn EJ, Park HT (2018) MicroRNA mediated regulation of schwann cell migration and proliferation in peripheral nerve injury. Biomed Res Int 2018:8198365
Landgraf P, Rusu M, Sheridan R et al (2007) A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129:1401–1414
Huang J, Wang J, Guo Q, Zou W (2019) Emerging roles of microRNAs in morphine tolerance. J Pain Res 12:1139–1147
Dai J, Dong R, Han X et al (2020) Osteoclast-derived exosomal let-7a-5p targets Smad2 to promote the hypertrophic differentiation of chondrocytes. Am J Physiol Cell Physiol 319(1):C21–C33
Duan S, Li J, Tian J et al (2019) Crosstalk between let-7a-5p and BCL-xL in the Initiation of Toxic Autophagy in Lung Cancer. Mol Ther Oncolytics 15:69–78
Duan S, Yu S, Yuan T, Yao S, Zhang L (2019) Exogenous Let-7a-5p induces A549 lung cancer cell death through BCL2L1-mediated PI3Kgamma signaling pathway. Front Oncol 9:808
Sommerkamp P, Renders S, Ladel L et al (2019) The long non-coding RNA Meg3 is dispensable for hematopoietic stem cells. Sci Rep 9:2110
Lee S, Seo HH, Lee CY et al (2017) Human long noncoding RNA regulation of stem cell potency and differentiation. Stem Cells Int 2017:6374504
Wang Q, Li Y, Zhang Y et al (2017) LncRNA MEG3 inhibited osteogenic differentiation of bone marrow mesenchymal stem cells from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed Pharmacother 89:1178–1186
Sun X, Luo LH, Feng L, Li DS (2018) Down-regulation of lncRNA MEG3 promotes endothelial differentiation of bone marrow derived mesenchymal stem cells in repairing erectile dysfunction. Life Sci 208:246–252
You D, Yang C, Huang J, Gong H, Yan M, Ni J (2019) Long non-coding RNA MEG3 inhibits chondrogenic differentiation of synovium-derived mesenchymal stem cells by epigenetically inhibiting TRIB2 via methyltransferase EZH2. Cell Signal 63:109379
Li Z, Jin C, Chen S et al (2017) Long non-coding RNA MEG3 inhibits adipogenesis and promotes osteogenesis of human adipose-derived mesenchymal stem cells via miR-140-5p. Mol Cell Biochem 433:51–60
Ghafouri-Fard S, Taheri M (2019) Maternally expressed gene 3 (MEG3): A tumor suppressor long non coding RNA. Biomed Pharmacother 118:109129
Funding
This work was supported by National Natural Science Foundation of China (Grant/Award Number: 81601083, 81702238, 81401006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, W., Gu, MF., Wang, ZF. et al. Let-7a-5p regulated by lncRNA-MEG3 promotes functional differentiation to Schwann cells from adipose derived stem cells via directly inhibiting RBPJ-mediating Notch pathway. Apoptosis 26, 548–560 (2021). https://doi.org/10.1007/s10495-021-01685-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-021-01685-x