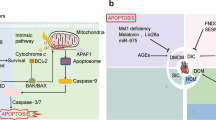
Abstract
Apoptosis repressor with caspase recruitment domain (ARC) is a highly effective and multifunctional inhibitor of apoptosis that is mainly expressed in postmitotic cells such as cardiomyocytes and skeletal muscle cells. ARC contains a C-terminal region rich in proline and glutamic acid residues and an N-terminal caspase recruitment domain (CARD). The CARD is originally described as a protein-binding motif that interacts with caspase through a CARD-CARD interaction. Initially, the inhibitory effect of ARC was only found in apoptosis, however, it was later found that ARC also played a regulatory role in other types of cell death. As a powerful cardioprotective factor, ARC can protect the heart by inhibiting the death of cardiomyocytes in various ways. ARC can reduce the cardiomyocyte apoptotic response to various stresses and injuries, including extrinsic apoptosis induced by death receptor ligands, cellular Ca2+ homeostasis and the dysregulation of endoplasmic reticulum (ER) stress, oxidative stress and hypoxia. In addition, changes in ARC transcription and translation levels in the heart can cause a series of physiological and pathological changes, and ARC can also perform corresponding functions through interactions with other molecules. Although there has been much research on ARC, the functional redundancy among proteins shows that ARC still has much research value. This review summarizes the molecular characteristics of ARC, its roles in the various death modes in cardiomyocytes and the roles of ARC in cardiac pathophysiology. This article also describes the potential therapeutic effect and research prospects of ARC.





Similar content being viewed by others
References
Bröker LE, Kruyt FA, Giaccone G (2005) Cell death independent of caspases: a review. Clin Cancer Res: Off J Am Assoc Cancer Res 11(9):3155–3162. https://doi.org/10.1158/1078-0432.ccr-04-2223
Fisher SA, Langille BL, Srivastava D (2000) Apoptosis during cardiovascular development. Circul Res 87(10):856–864. https://doi.org/10.1161/01.res.87.10.856
Yuan J, Kroemer G (2010) Alternative cell death mechanisms in development and beyond. Genes Dev 24(23):2592–2602. https://doi.org/10.1101/gad.1984410
Barbosa LA, Fiuza PP, Borges LJ, Rolim FA, Andrade MB, Luz NF, Quintela-Carvalho G, Lima JB, Almeida RP, Chan FK, Bozza MT, Borges VM, Prates DB (2018) RIPK1-RIPK3-MLKL-associated necroptosis drives leishmania infantum killing in neutrophils. Front Immunol 9:1818. https://doi.org/10.3389/fimmu.2018.01818
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7(2):99–109. https://doi.org/10.1038/nrmicro2070
Koseki T, Inohara N, Chen S, Nunez G (1998) ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci USA 95(9):5156–5160. https://doi.org/10.1073/pnas.95.9.5156
Stoss O, Schwaiger F-W, Cooper TA, Stamm S (1999) Alternative splicing determines the intracellular localization of the novel nuclear protein Nop30 and its interaction with the splicing factor SRp30c. J Biol Chem 274(16):10951–10962
Nam YJ, Mani K, Ashton AW, Peng CF, Kitsis RN (2004) Inhibition of both the extrinsic and intrinsic death pathways through nonhomotypic death-fold interactions. Mol Cell 15(6):901–912
Zhu S, Zhang Z, Jia LQ, Zhan KX, Wang LJ, Song N, Liu Y, Cheng YY, Yang YJ, Guan L, Min DY, Yang GL (2019) Valproic acid attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-pyroptosis pathways. Neurochem Int 124:141–151. https://doi.org/10.1016/j.neuint.2019.01.003
Tao Xu, Wei Ding, Xiang Ao, Xianming Chu, Qinggong Wan (2019) ARC regulates programmed necrosis and myocardial ischemia/reperfusion injury through the inhibition of mPTP opening. Redox Biol 20:414–426. https://doi.org/10.1016/j.redox.2018.10.023
Balakumar P, Maung UK, Jagadeesh G (2016) Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res 113(Pt A):600–609. https://doi.org/10.1016/j.phrs.2016.09.040
Li X, Du N, Zhang Q, Li J, Chen X, Liu X, Hu Y, Qin W, Shen N, Xu C (2014) MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis 5(10):e1479. https://doi.org/10.1038/cddis.2014.430
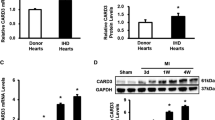
Li J, Li C, Zhang D, Shi D, Qi M, Feng J, Yuan T, Xu X, Liang D, Xu L (2014) SNX13 reduction mediates heart failure through degradative sorting of apoptosis repressor with caspase recruitment domain. Nat Commun 5:5177. https://doi.org/10.1038/ncomms6177
Chatterjee S, Bish LT, Jayasankar V, Stewart AS, Sweeney HL (2003) Blocking the development of postischemic cardiomyopathy with viral gene transfer of the apoptosis repressor with caspase recruitment domain. J Thorac Cardiovasc Surg 125(6):1461–1469. https://doi.org/10.1016/s0022-5223(02)73229-7
Murtaza I, Wang H-X, Feng X, Alenina N, Bader M, Prabhakar BS, Li P-F (2008) Down-regulation of catalase and oxidative modification of protein kinase CK2 lead to the failure of apoptosis repressor with caspase recruitment domain to inhibit cardiomyocyte hypertrophy. J Biol Chem 283(10):5996–6004. https://doi.org/10.1074/jbc.M706466200
An J, Li P, Li J, Dietz R, Donath S (2009) ARC is a critical cardiomyocyte survival switch in doxorubicin cardiotoxicity. J Mol Med (Berlin, Germany) 87(4):401–410. https://doi.org/10.1007/s00109-008-0434-z
Geertman R, Mcmahon A, Sabban EL (1996) Cloning and characterization of cDNAs for novel proteins with glutamic acid-proline dipeptide tandem repeats. Biochim Biophys Acta 1306(2–3):147–152. https://doi.org/10.1016/0167-4781(96)00036-x
Abmayr S (2004) Characterization of ARC, apoptosis repressor interacting with CARD, in normal and dystrophin-deficient skeletal muscle. Human Mol Genet 13(2):213–221. https://doi.org/10.1093/hmg/ddh018
Engidawork E, Gulesserian T, Yoo BC, Cairns N, Lubec G (2001) Alteration of caspases and apoptosis-related proteins in brains of patients with Alzheimer’s disease. Biochem Biophys Res Commun 281(1):93. https://doi.org/10.1006/bbrc.2001.4306
Quadrilatero J, Bloemberg D (2010) Apoptosis repressor with caspase recruitment domain is dramatically reduced in cardiac, skeletal, and vascular smooth muscle during hypertension. Biochem Biophys Res Commun 391(3):1442. https://doi.org/10.1016/j.bbrc.2009.12.084
Jewgenow K, Neubauer K, Blottner S, Schon J, Wildt DE, Pukazhenthi BS (2009) Reduced germ cell apoptosis during spermatogenesis in the teratospermic domestic cat. J Androl 30(4):460–468. https://doi.org/10.2164/jandrol.108.006726
Rimon E, Sasson R, Dantes A, Landbracha A, Amsterdam A (2004) Gonadotropin-induced gene regulation in human granulosa cells obtained from IVF patients: modulation of genes coding for growth factors and their receptors and genes involved in cancer and other diseases. International journal of oncology 24(5):1325. https://doi.org/10.3892/ijo.24.5.1325
Mckimpson WM, Weinberger J, Czerski L, Zheng M, Kitsis RN (2013) The apoptosis inhibitor ARC alleviates the ER stress response to promote β-cell survival. Diabetes 62(1):183–193. https://doi.org/10.2337/db12-0504
Guan M, Fang Q, He Z, Li Y, Chai R (2016) Inhibition of ARC decreases the survival of HEI-OC-1 cells after neomycin damage in vitro. Oncotarget 7(41):66647–66659. https://doi.org/10.18632/oncotarget.11336
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91(4):479–489. https://doi.org/10.1016/s0092-8674(00)80434-1
Bouchier-Hayes L, Martin SJ (2002) CARD games in apoptosis and immunity. EMBO Rep 3(7):616–621. https://doi.org/10.1093/embo-reports/kvf139
Hua Z, Li Y, Liu X, Wang X (1999) An APAF-1·Cytochrome c Multimeric Complex Is a Functional Apoptosome That Activates Procaspase-9 *. J Biol Chem 274(17):11549–11556. https://doi.org/10.1074/jbc.274.17.11549
Tinel A, Tschopp J (2004) The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304(5672):843–846. https://doi.org/10.1126/science.1095432
Jo DG, Jun J-I, Chang J-W, Hong Y-M, Song S, Cho D-H, Shim SM, Lee H-J, Cho C, Kim DH (2004) Calcium binding of ARC mediates regulation of caspase 8 and cell death. Mol Cell Biol 24(22):9763–9770. https://doi.org/10.1128/mcb.24.22.9763-9770.2004
Foo RS, Nam YJ, Ostreicher MJ, Metzl MD, Whelan RS, Peng CF, Ashton AW, Fu W, Mani K, Chin SF, Provenzano E, Ellis I, Figg N, Pinder S, Bennett MR, Caldas C, Kitsis RN (2007) Regulation of p53 tetramerization and nuclear export by ARC. Proc Natl Acad Sci USA 104(52):20826–20831. https://doi.org/10.1073/pnas.0710017104
Kim SH, Park HH (2015) Crystallization and preliminary X-ray crystallographic analysis of the CARD domain of apoptosis repressor with CARD (ARC). Acta Crystallographica Sect F, Struct Biol Commun 71(Pt 1):82–85. https://doi.org/10.1107/S2053230X14026211
Wu L, Nam Y-J, Kung G, Crow MT, Kitsis RN (2010) Induction of the apoptosis inhibitor ARC by ras in human cancers. J Biol Chem 285(25):19235–19245. https://doi.org/10.1074/jbc.M110.114892
Li YZ, Lu D-Y, Tan W-Q, Wang J-X, Li P-F (2008) p53 initiates apoptosis by transcriptionally targeting the antiapoptotic protein ARC. Mol Cell Biol 28(2):564–574. https://doi.org/10.1128/mcb.00738-07
Li Y, Liu X, Rong F (2011) Puma mediates the apoptotic signal of hypoxia/reoxygenation in cardiomyocytes through mitochondrial pathway. Shock 35(6):579–584. https://doi.org/10.1097/SHK.0b013e318211601a
Lu D, Liu J, Jiao J, Long B, Li Q, Tan W, Li P (2013) Transcription factor Foxo3a prevents apoptosis by regulating calcium through the apoptosis repressor with caspase recruitment domain. J Biol Chem 288(12):8491–8504
Li Q, Wang J-X, He Y-Q, Feng C, Zhang X-J, Sheng J-Q, Li P-F (2014) MicroRNA-185 regulates chemotherapeutic sensitivity in gastric cancer by targeting apoptosis repressor with caspase recruitment domain. Cell Death Dis 5(4):e1197. https://doi.org/10.1038/cddis.2014.148
Wang J-X, Zhang X-J, Feng C, Sun T, Wang K, Wang Y, Zhou L-Y, Li P-F (2015) MicroRNA-532-3p regulates mitochondrial fission through targeting apoptosis repressor with caspase recruitment domain in doxorubicin cardiotoxicity. Cell Death Dis 6(3):e1677. https://doi.org/10.1038/cddis.2015.41
Wu H, Huang T, Ying L, Han C, Li D, Xu Y, Zhang M, Mou S, Dong Z (2016) MiR-155 is involved in renal ischemia-reperfusion injury via direct targeting of FoxO3a and regulating renal tubular cell pyroptosis. Cell Physiol Biochem: Int J Exp Cell Physiol Biochem Pharmacol 40(6):1692–1705. https://doi.org/10.1159/000453218
Li Q, Yang J, Zhang J, Liu XW, Yang CJ, Fan ZX, Wang HB, Yang Y, Zheng T, Yang J (2020) Inhibition of microRNA-327 ameliorates ischemia/reperfusion injury-induced cardiomyocytes apoptosis through targeting apoptosis repressor with caspase recruitment domain. J Cell Physiolo 235(4):3753–3767. https://doi.org/10.1002/jcp.29270
Foo SY, Chan LKW, Kitsis RN, Bennett MR (2007) Ubiquitination and degradation of the anti-apoptotic protein ARC by MDM2. J Biol Chem 282(8):5529–5535. https://doi.org/10.1074/jbc.M609046200
Nam YJ, Mani K, Wu L, Peng CF, Calvert JW, Foo RS, Krishnamurthy B, Miao W, Ashton AW, Lefer DJ, Kitsis RN (2007) The apoptosis inhibitor ARC undergoes ubiquitin-proteasomal-mediated degradation in response to death stimuli: identification of a degradation-resistant mutant. J Biol Chem 282(8):5522–5528. https://doi.org/10.1074/jbc.M609186200
Haupt Y, Maya R, Kazaz A, Oren M (1997) Mdm2 promotes the rapid degradation of p53. Nature 387(6630):296–299. https://doi.org/10.1038/387296a0
Kubbutat MH, Jones SN, Vousden KH (1997) Regulation of p53 stability by Mdm2. Nature 387(6630):299–303. https://doi.org/10.1038/387299a0
Loan Le TY, Mardini M, Howell VM, Funder JW, Ashton AW, Mihailidou AS (2012) Low-dose spironolactone prevents apoptosis repressor with caspase recruitment domain degradation during myocardial infarction. Hypertension 59(6):1164–1169. https://doi.org/10.1161/hypertensionaha.111.190488
Kavazis AN, McClung JM, Hood DA, Powers SK (2008) Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294(2):H928-935. https://doi.org/10.1152/ajpheart.01231.2007
Li PF, Li J, Müller E-C, Otto A, Dietz R, Rv Harsdorf (2002) Phosphorylation by protein kinase CK2: a signaling switch for the caspase-inhibiting protein ARC. Molecular Cell 10(2):247–258. https://doi.org/10.1016/s1097-2765(02)00600-7
Tan WQ, Wang J-X, Lin Z-Q, Li Y-R, Lin Y, Li P-F (2008) Novel cardiac apoptotic pathway: the dephosphorylation of apoptosis repressor with caspase recruitment domain by calcineurin. Circulation 118(22):2268–2276. https://doi.org/10.1161/circulationaha.107.750869
Lu X, Moore PG, Liu H, Schaefer S (2011) Phosphorylation of ARC is a critical element in the antiapoptotic effect of anesthetic preconditioning. Anesthesia Analgesia 112(3):525–531. https://doi.org/10.1213/ANE.0b013e318205689b
Yao X, Tan G, He C, Gao Y, Pan S, Jiang H, Zhang Y, Sun X (2012) Hydrogen sulfide protects cardiomyocytes from myocardial ischemia-reperfusion injury by enhancing phosphorylation of apoptosis repressor with caspase recruitment domain. Tohoku J Exp Med 226(4):275–285. https://doi.org/10.1620/tjem.226.275
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2(4):277–288. https://doi.org/10.1038/nrc776
Tait SW, Green DR (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11(9):621–632. https://doi.org/10.1038/nrm2952
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116(2):205–219. https://doi.org/10.1016/s0092-8674(04)00046-7
Itoh N, Nagata S (1993) A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem 268(15):10932–10937
Donath S, Li P, Willenbockel C, Al-Saadi N, Gross V, Willnow T, Bader M, Martin U, Bauersachs J, Wollert KC, Dietz R, von Harsdorf R (2006) Apoptosis repressor with caspase recruitment domain is required for cardioprotection in response to biomechanical and ischemic stress. Circulation 113(9):1203–1212. https://doi.org/10.1161/circulationaha.105.576785
Ekhterae D, Platoshyn O, Zhang S, Remillard CV, Yuan JX (2003) Apoptosis repressor with caspase domain inhibits cardiomyocyte apoptosis by reducing K+ currents. Am J Physiol Cell Physiol 284(6):C1405–C1410. https://doi.org/10.1152/ajpcell.00279.2002
Brenner D, Mak TW (2009) Mitochondrial cell death effectors. Curr Opin Cell Biol 21(6):871–877. https://doi.org/10.1016/j.ceb.2009.09.004
Wang JX, Li Q, Li PF (2009) Apoptosis repressor with caspase recruitment domain contributes to chemotherapy resistance by abolishing mitochondrial fission mediated by dynamin-related protein-1. Cancer Res 69(2):492–500. https://doi.org/10.1158/0008-5472.can-08-2962
Ekhterae D, Lin Z, Lundberg MS, Crow MT, Brosius FC, Nunez G (1999) ARC inhibits cytochrome c release from mitochondria and protects against hypoxia-induced apoptosis in heart-derived H9c2 cells. Circul Res 85(12):e70–e77. https://doi.org/10.1161/01.res.85.12.e70
Yu ZL, Xiu HL, Xiao MZ, Li RC (2007) ARC contributes to the inhibitory effect of preconditioning on cardiomyocyte apoptosis. Apoptosis: Int J Programm Cell Death 12(9):1589–1595. https://doi.org/10.1007/s10495-007-0094-4
Li YZ, Ge X, Liu X (2009) The cardioprotective effect of postconditioning is mediated by ARC through inhibiting mitochondrial apoptotic pathway. Apoptosis: Int J Programm Cell Death 14(2):164–172. https://doi.org/10.1007/s10495-008-0296-4
Neuss M, Monticone R, Lundberg MS, Chesley AT, Fleck E, Crow MT (2001) The apoptotic regulatory protein ARC (Apoptosis Repressor with Caspase Recruitment Domain) prevents oxidant stress-mediated cell death by preserving mitochondrial function. J Biol Chem 276(36):33915–33922. https://doi.org/10.1074/jbc.M104080200
Gustafsson AB, Sayen MR, Williams SD, Crow MT, Gottlieb RA (2002) TAT protein transduction into isolated perfused hearts: TAT-apoptosis repressor with caspase recruitment domain is cardioprotective. Circulation 106(6):735–739. https://doi.org/10.1161/01.cir.0000023943.50821.f7
Gustafsson AB, Tsai JG, Logue SE, Crow MT, Gottlieb RA (2004) Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with bax activation. J Biol Chem 279(20):21233–21238. https://doi.org/10.1074/jbc.M400695200
Li J, Li Y, Qin D, von Harsdorf R, Li P (2010) Mitochondrial fission leads to Smac/DIABLO release quenched by ARC. Apoptosis: Int J Programm Cell Death 15(10):1187–1196. https://doi.org/10.1007/s10495-010-0514-8
Yu J, Wang P, Ming L, Wood MA, Zhang L (2007) SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene 26(29):4189–4198. https://doi.org/10.1038/sj.onc.1210196
Boyce M, Yuan J (2006) Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Different 13(3):363–373. https://doi.org/10.1038/sj.cdd.4401817
Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Ann Rev Biochem 74:739–789. https://doi.org/10.1146/annurev.biochem.73.011303.074134
Li HC, Chen CJ, Watts R et al (2008) Inhibition of endoplasmic reticulum stress-induced apoptosis of melanoma cells by the ARC protein. Cancer Res 68(3):834. https://doi.org/10.1158/0008-5472.can-07-5056
McKimpson WM, Weinberger J, Czerski L, Zheng M, Crow MT, Pessin JE, Chua SC Jr, Kitsis RN (2013) The apoptosis inhibitor ARC alleviates the ER stress response to promote β-cell survival. Diabetes 62(1):183–193. https://doi.org/10.2337/db12-0504
Schroder M, Kaufman RJ (2005) ER stress and the unfolded protein response. Mutat Res 569(1–2):29–63. https://doi.org/10.1016/j.mrfmmm.2004.06.056
Pyo JO, Nah J, Kim HJ, Chang JW, Song YW, Yang DK, Jo DG, Kim HR, Chae HJ, Chae SW, Hwang SY, Kim SJ, Kim HJ, Cho C, Oh CG, Park WJ, Jung YK (2008) Protection of cardiomyocytes from ischemic/hypoxic cell death via Drbp1 and pMe2GlyDH in cardio-specific ARC transgenic mice. J Biol Chem 283(45):30707–30714. https://doi.org/10.1074/jbc.M804209200
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112–119. https://doi.org/10.1038/nchembio711
Christofferson DE, Yuan J (2010) Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22(2):263–268. https://doi.org/10.1016/j.ceb.2009.12.003
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434(7033):652–658. https://doi.org/10.1038/nature03317
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11(10):700–714. https://doi.org/10.1038/nrm2970
Yaglom JA, Ekhterae D, Gabai VL, Sherman MY (2003) Regulation of necrosis of H9c2 myogenic cells upon transient energy deprivation Rapid deenergization of mitochondria precedes necrosis and is controlled by reactive oxygen species, stress kinase JNK, HSP72 and ARC. J Biol Chem 278(50):50483–50496. https://doi.org/10.1074/jbc.M306903200
An J, Harms C, Lättig-Tünnemann G, Sellge G, Mandić AD, Malato Y, Heuser A, Endres M, Trautwein C, Donath S (2012) TAT-apoptosis repressor with caspase recruitment domain protein transduction rescues mice from fulminant liver failure. Hepatology. https://doi.org/10.1002/hep.25697
An J, Mehrhof F, Harms C, Lättig-Tünnemann G, Lee SL, Endres M, Li M, Sellge G, Mandić AD, Trautwein C, Donath S (2013) ARC is a novel therapeutic approach against acetaminophen-induced hepatocellular necrosis. J Hepatol 58(2):297–305. https://doi.org/10.1016/j.jhep.2012.10.002
Kung G, Dai P, Deng L, Kitsis RN (2014) A novel role for the apoptosis inhibitor ARC in suppressing TNFα-induced regulated necrosis. Cell Death Different 21(4):634–644. https://doi.org/10.1038/cdd.2013.195
Wang Y, Zhao M, He S, Luo Y, Zhao Y, Cheng J, Gong Y, Xie J, Wang Y, Hu B, Tian L, Liu X, Li C, Huang Q (2019) Necroptosis regulates tumor repopulation after radiotherapy via RIP1/RIP3/MLKL/JNK/IL8 pathway. J Exp Clin Cancer Res. https://doi.org/10.1186/s13046-019-1423-5
Zhou C, Chen Z, Lu X, Wu H, Yang Q, Xu D (2016) Icaritin activates JNK-dependent mPTP necrosis pathway in colorectal cancer cells. Tumor Biol 37(3):3135–3144. https://doi.org/10.1007/s13277-015-4134-3
Cookson BT, Brennan MA (2001) Pro-inflammatory programmed cell death. Trends Microbiol 9(3):113–114. https://doi.org/10.1016/s0966-842x(00)01936-3
Siu PM, Bryner RW, Murlasits Z, Alway SE (2005) Response of XIAP, ARC, and FLIP apoptotic suppressors to 8 wk of treadmill running in rat heart and skeletal muscle. J Appl Physiol 99(1):204–209. https://doi.org/10.1152/japplphysiol.00084.2005
Li Q, Ren J (2007) Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell 6(6):799–806. https://doi.org/10.1111/j.1474-9726.2007.00343.x
Kurian GA, Rajagopal R, Vedantham S, Rajesh M (2016) The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Oxidat Med Cell Longev 2016:1–14. https://doi.org/10.1155/2016/1656450
Granger DN, Kvietys PR (2015) Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol 6:524–551. https://doi.org/10.1016/j.redox.2015.08.020
Murata M, Akao M, O’Rourke B, Marban E (2001) Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca(2+) overload during simulated ischemia and reperfusion: possible mechanism of cardioprotection. Circulation Res 89(10):891–898. https://doi.org/10.1161/hh2201.100205
Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL (2017) Mitochondrial dysfunction and myocardial ischemia-reperfusion: implications for novel therapies. Ann Rev Pharmacol 57(1):535–565. https://doi.org/10.1146/annurev-pharmtox-010715-103335
Ekhterae D, Hinmon R, Matsuzaki K, Noma M, Zhu W, Xiao RP, Gorman RC, Gorman JH 3rd (2011) Infarction induced myocardial apoptosis and ARC activation. J Surg Res 166(1):67. https://doi.org/10.1016/j.jss.2009.05.002
Bouma W, Noma M, Kanemoto S, Matsubara M, Leshnower BG, Hinmon R, Gorman JH 3rd, Gorman RC (2010) Sex-related resistance to myocardial ischemia-reperfusion injury is associated with high constitutive ARC expression. Am J Physiol Heart Circ Physiol 298(5):H1510. https://doi.org/10.1152/ajpheart.01021.2009
Xie F, Mei ZS, Wang X, Zhang T, Zhao Y, Wang SD, Qian LJ (2020) Loss of nuclear ARC contributes to the development of cardiac hypertrophy in rats. Acta physiologica (Oxford, England) 228(2):e13337. https://doi.org/10.1111/apha.13337
Litwin SE (2013) Diabetes and the heart: is there objective evidence of a human diabetic cardiomyopathy? Diabetes 62(10):3329–3330. https://doi.org/10.2337/db13-0683
Mohammadshahi M, Haidari F, Soufi FG (2014) Chronic resveratrol administration improves diabetic cardiomyopathy in part by reducing oxidative stress. Cardiol J 21(1):39. https://doi.org/10.5603/CJ.a2013.0051
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321(7258):405–412. https://doi.org/10.1136/bmj.321.7258.405
Wen HL, Liang ZS, Zhang R, Yang K (2013) Anti-inflammatory effects of triptolide improve left ventricular function in a rat model of diabetic cardiomyopathy. Cardiovasc Diabetol 12(1):50. https://doi.org/10.1186/1475-2840-12-50
Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. New England J Med 339(13):900–905. https://doi.org/10.1056/nejm199809243391307
Otto A, Stähle I, Klein R, Berg PA, Pankuweit S, Brandsch R (1998) Anti-mitochondrial antibodies in patients with dilated cardiomyopathy (anti-M7) are directed against flavoenzymes with covalently bound FAD. Clin Exp Immunol 111(3):541–547. https://doi.org/10.1046/j.1365-2249.1998.00531.x
Kitano H (2004) Biological robustness - a principle in systems biology. Nat Rev Genet 5(11):826–837
Funding
This work was funded by Natural Science Foundation of Shandong Province (JQ201815).
Author information
Authors and Affiliations
Contributions
WD established the main idea of the manuscript. JZ and XZ wrote the manuscript and prepared the figures. PW and WD contributed with new ideas and references of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Ethics approval
Compliance with ethical standards
Consent to participate
The authors agree to participate.
Consent for publication
The authors agree to publications.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Zheng, X., Wang, P. et al. Role of apoptosis repressor with caspase recruitment domain (ARC) in cell death and cardiovascular disease. Apoptosis 26, 24–37 (2021). https://doi.org/10.1007/s10495-020-01653-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-020-01653-x