Abstract
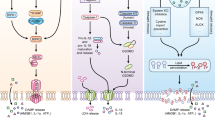
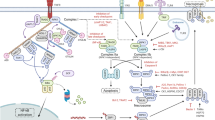
Necroptosis is a noncaspase-dependent and precisely regulated mechanism of cell death. Necroptosis is mainly initiated by members of the tumor necrosis factor receptor (TNFR) and Toll-like receptor (TLR) families, interferon, intracellular RNA and DNA sensors and other mediators. Subsequently, the protein kinase RIPK1 (receptor-interacting protein kinase 1) and RIPK3 interact with the receptor protein, which transduces death signals and further recruits and phosphorylates MLKL (mixed lineage kinase domain-like protein). MLKL serves as the initiator of cell death and eventually induces necroptosis. It was found that necroptosis is not only involved in the physiological regulation but also in the occurrence, development and prognosis of some necrotic diseases, especially infectious diseases. Intervention in the necroptosis signaling pathway is helpful for removing pathogens, inhibiting the development of lesions, and promoting the remodeling of tissue. In-depth study of the molecular regulation mechanism of necroptosis and its relationship with the pathogenesis of infectious diseases will help to provide new ideas and directions for research of the pathological mechanisms and clinical prevention of infectious diseases.



Similar content being viewed by others
References
Kroemer G, Galluzzi L, Vandenabeele P et al (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16(1):3. https://doi.org/10.1038/sj.cdd.4401724
Galluzzi L, Vitale I, Aaronson SA et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25(3):486–541. https://doi.org/10.1038/s41418-017-0012-4
Melino G (2001) The sirens' song. Nature 412(6842):23
Galluzzi L, Kroemer G (2008) Necroptosis: a specialized pathway of programmed necrosis. Cell 135(7):1161–1163. https://doi.org/10.1016/j.cell.2008.12.004
Galluzzi L, Vitale I, Abrams JM et al (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19(1):107–120. https://doi.org/10.1038/cdd.2011.96
Galluzzi L, Kepp O, Krautwald S, et al. (2014) Molecular mechanisms of regulated necrosis. In: Seminars in cell & developmental biology. Academic Press, Massachusetts.
Moriwaki K, Balaji S, McQuade T et al (2014) The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity 41(4):567–578. https://doi.org/10.1016/j.immuni.2014.09.016
Upton JW, Kaiser WJ, Mocarski ES (2012) DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11(3):290–297. https://doi.org/10.1016/j.chom.2012.01.016
Oberst A, Green DR (2011) It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol 12(11):757. https://doi.org/10.1038/nrm3214
Kaiser WJ, Upton JW, Long AB et al (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471(7338):368–372. https://doi.org/10.1038/nature09857
Holler N, Zaru R, Micheau O et al (2000) Fas triggers an alternative, caspase-8–independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1(6):489. https://doi.org/10.1021/la7016177
Zhao J, Jitkaew S, Cai Z et al (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA 109(14):5322–5327. https://doi.org/10.1073/pnas.1200012109
Cho Y, Challa S, Moquin D et al (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123. https://doi.org/10.1016/j.cell.2009.05.037
He S, Wang L, Miao L et al (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137(6):1100–1111. https://doi.org/10.1016/j.cell.2009.05.021
Zhang DW, Shao J, Lin J et al (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325(5938):332–336. https://doi.org/10.1126/science.1172308
Sun L, Wang H, Wang Z et al (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148(1–2):213–227. https://doi.org/10.1016/j.cell.2011.11.031
Wang X, Li Y, Liu S et al (2014) Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci USA 111(43):15438–15443. https://doi.org/10.1073/pnas.1412767111
Golstein P, Kroemer G (2007) Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 32(1):37–43. https://doi.org/10.1016/j.tibs.2006.11.001
He S, Liang Y, Shao F, Wang X (2011) Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3–mediated pathway. Proc Natl Acad Sci USA 108(50):20054–20059. https://doi.org/10.1073/pnas.1116302108
Chen X, Li W, Ren J et al (2014) Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 24(1):105. https://doi.org/10.1038/cr.2013.171
Pearson JS, Giogha C, Ong SY et al (2013) A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature 501(7466):247. https://doi.org/10.1038/nature12524
Laster SM, Wood JG, Gooding LR (1988) Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol 141(8):2629–2634
Macen J, Takahashi A, Moon KB et al (1998) Activation of caspases in pig kidney cells infected with wild-type and CrmA/SPI-2 mutants of cowpox and rabbitpox viruses. J Virol 72(5):3524–3533. https://doi.org/10.1016/S0166-0934(98)00009-3
Vercammen D, Beyaert R, Denecker G et al (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 187(9):1477–1485. https://doi.org/10.1016/j.chom.2010.03.006
Chan FKM, Shisler J, Bixby JG et al (2003) A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem 278(51):51613–51621. https://doi.org/10.1074/jbc.M305633200
Degterev A, Huang Z, Boyce M et al (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112. https://doi.org/10.1038/nchembio711
Wu W, Liu P, Li J (2012) Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol/Hematol 82(3):249–258. https://doi.org/10.1016/j.critrevonc.2011.08.004
Linkermann A, Hackl MJ, Kunzendorf U et al (2013) Necroptosis in immunity and ischemia-reperfusion injury. Am J Transpl 13(11):2797–2804. https://doi.org/10.1111/ajt.12448
He S, Huang S, Shen Z (2016) Biomarkers for the detection of necroptosis. Cell Mol Life Sci 73(11–12):2177–2181. https://doi.org/10.1007/s00018-016-2192-3
Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517(7534):311–320. https://doi.org/10.1038/nature14191
de Almagro MC, Vucic D (2015) Necroptosis: pathway diversity and characteristics. In: Seminars in cell & developmental biology. Academic Press, Massachusetts.
Blaser H, Dostert C, Mak TW, Brenner D (2016) TNF and ROS crosstalk in inflammation. Trends Cell Biol 26(4):249–261. https://doi.org/10.1016/j.tcb.2015.12.002
Kearney CJ, Martin SJ (2017) An inflammatory perspective on necroptosis. Mol Cell 65(6):965–973. https://doi.org/10.1016/j.molcel.2017.02.024
Galluzzi L, Kepp O, Chan FKM, Kroemer G (2017) Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol 12:103–130. https://doi.org/10.1146/annurev-pathol-052016-100247
Martinon F, Gaide O, Pétrilli V, Mayor A, Tschopp J (2007) NALP inflammasomes: a central role in innate immunity. In: Seminars in immunopathology. Springer-Verlag, Berlin.
Newton K, Manning G (2016) Necroptosis and inflammation. Annu Rev Biochem 85:743–763. https://doi.org/10.1146/annurev-biochem-060815-014830
Kim SJ, Lee SM (2017) Necrostatin-1 protects against d-Galactosamine and lipopolysaccharide-induced hepatic injury by preventing TLR4 and RAGE signaling. Inflammation 40(6):1912–1923. https://doi.org/10.1007/s10753-017-0632-3
Lawlor KE, Khan N, Mildenhall A et al (2015) RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun 6:6282. https://doi.org/10.1038/ncomms7282
Kaiser WJ, Sridharan H, Huang C et al (2013) Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288(43):31268–31279. https://doi.org/10.1074/jbc.M113.462341
Osborn SL, Diehl G, Han SJ et al (2010) Fas-associated death domain (FADD) is a negative regulator of T-cell receptor–mediated necroptosis. Proc Natl Acad Sci USA 107(29):13034–13039. https://doi.org/10.1073/pnas.1005997107
Stanger BZ, Leder P, Lee TH, Kim E, Seed B (1995) RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81(4):513–523. https://doi.org/10.1016/0092-8674(95)90072-1
Kelliher MA, Grimm S, Ishida Y, Kuo F, Leder SBZ, P (1998) The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8(3):297–303. https://doi.org/10.1016/S1074-7613(00)80535-X
Christofferson DE, Yuan J (2010) Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22(2):263–268. https://doi.org/10.1016/j.ceb.2009.12.003
Lee EW, Seo JH, Jeong MH, Lee SS, Song JW (2012) The roles of FADD in extrinsic apoptosis and necroptosis. BMB Rep 45(9):496–508. https://doi.org/10.5483/BMBRep.2012.45.9.186
Kaiser WJ, Daley-Bauer LP, Thapa RJ et al (2014) RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci USA 111(21):7753–7758. https://doi.org/10.1073/pnas.1401857111
Dannappel M, Vlantis K, Kumari S et al (2014) RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513(7516):90. https://doi.org/10.1038/nature13608
Wagner PN, Shi Q, Salisbury-Ruf CT et al (2019) Increased Ripk1-mediated bone marrow necroptosis leads to myelodysplasia and bone marrow failure in mice. Blood 133(2):107–120. https://doi.org/10.1182/blood-2018-05-847335
Takahashi N, Vereecke L, Bertrand MJ et al (2014) RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 513(7516):95–99. https://doi.org/10.1038/nature13706
Berger SB, Kasparcova V, Hoffman S et al (2014) Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol 192(12):5476–5480. https://doi.org/10.4049/jimmunol.1400499
Newton K, Dugger DL, Wickliffe KE et al (2014) Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343(6177):1357–1360. https://doi.org/10.1126/science.1249361
Narayan N, Lee IH, Borenstein R et al (2012) The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature 492(7428):199. https://doi.org/10.1038/nature12897
Trichonas G, Murakami Y, Thanos A et al (2010) Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci USA 107(50):21695–21700. https://doi.org/10.1073/pnas.1009179107
Hildebrand JM, Lucet IS, Murphy JM (2015) Flicking the molecular switch underlying MLKL-mediated necroptosis. Mol Cell Oncol 2(3):e985550. https://doi.org/10.4161/23723556.2014.985550
Zhang J, Yang Y, He W, Sun L (2016) Necrosome core machinery: MLKL. Cell Mol Lif Sci 73(11–12):2153–2163. https://doi.org/10.1007/s00018-016-2190-5
Xia B, Fang S, Chen X et al (2016) MLKL forms cation channels. Cell Res 26(5):517. https://doi.org/10.1038/cr.2016.26
Kim SK, Yun M, Seo G, et al. (2017) Palmitate induces RIP1/RIP3-dependent necrosis via MLKL-mediated pore formation in the plasma membrane of RAW 264.7 cells. Biochem Biophys Res Commun 482(2), 359–365 Doi: 10.1016/j.bbrc.2016.11.068.
Cai Z, Jitkaew S, Zhao J et al (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16(1):55. https://doi.org/10.1038/ncb2883
Wu J, Huang Z, Ren J et al (2013) Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res 23(8):994. https://doi.org/10.1038/cr.2013.91
Upton JW, Kaiser WJ, Mocarski ES (2008) Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem 283(25):16966–16970. https://doi.org/10.1074/jbc.c800051200
Lin J, Kumari S, Kim C et al (2016) RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540(7631):124. https://doi.org/10.1038/nature20558
Pham CL, Shanmugam N, Strange M et al (2019) Viral M45 and necroptosis-associated proteins form heteromeric amyloid assemblies. EMBO Rep. https://doi.org/10.15252/embr.201846518
Aravalli RN, Hu S, Lokensgard JR (2008) Inhibition of toll-like receptor signaling in primary murine microglia. J Neuroimmune Pharmacol 3(1):5–11. https://doi.org/10.1007/s11481-007-9097-8
Bowie AG, Unterholzner L (2008) Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol 8(12):911. https://doi.org/10.1038/nri2436
Guo H, Omoto S, Harris PA et al (2015) Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe 17(2):243–251. https://doi.org/10.1016/j.chom.2015.01.003
Huang Z, Wu SQ, Liang Y et al (2015) RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe 17(2):229–242. https://doi.org/10.1016/j.chom.2015.01.002
Nogusa S, Thapa RJ, Dillon CP et al (2016) RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza A virus. Cell Host Microbe 20(1):13–24. https://doi.org/10.1016/j.chom.2016.05.011
Gaba A, Xu F, Lu Y et al (2019) The NS1 protein of influenza A virus participates in necroptosis by interacting with MLKL and increasing its oligomerization and membrane translocation. J Virol 93(2):e01835–e1918. https://doi.org/10.1128/JVI.01835-18
Li S, Zhang L, Yao Q et al (2013) Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature 501(7466):242. https://doi.org/10.1038/nature12436
Weng D, Marty-Roix R, Ganesan S et al (2014) Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci USA 111(20):7391–7396. https://doi.org/10.1073/pnas.1403477111
Philip NH, Dillon CP, Snyder AG et al (2014) Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling. Proc Natl Acad Sci USA 111(20):7385–7390. https://doi.org/10.1073/pnas.1403252111
Blériot C, Lecuit M (2016) The interplay between regulated necrosis and bacterial infection. Cell Mol Life Sci 73(11–12):2369–2378. https://doi.org/10.1007/s00018-016-2206-1
Robinson N, McComb S, Mulligan R et al (2012) Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol 13(10):954. https://doi.org/10.1038/ni.2397
Hu GQ, Yang YJ, Qin XX et al (2019) Salmonella outer protein B suppresses colitis development via protecting cell from necroptosis. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2019.00087
Ro YT, Jo GH, Jung, et al (2018) Salmonella-induced miR-155 enhances necroptotic death in macrophage cells via targeting RIP1/3. Mol Med Rep 18(6):5133–5140. https://doi.org/10.3892/mmr.2018.9525
Kothari H, Keshava S, Vatsyayan R et al (2014) Role of tissue factor in Mycobacterium tuberculosis-induced inflammation and disease pathogenesis. PLoS ONE 9(12):e114141. https://doi.org/10.1371/journal.pone.0114141
Sridharan H, Upton JW (2014) Programmed necrosis in microbial pathogenesis. Trends Microbiol 22(4):199–207. https://doi.org/10.1016/j.tim.2014.01.005
Kitur K, Parker D, Nieto P et al (2015) Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog 11(4):e1004820. https://doi.org/10.1371/journal.ppat.1004820
Morinaga Y, Yanagihara K, Nakamura S et al (2010) Legionella pneumophila induces cathepsin B-dependent necrotic cell death with releasing high mobility group box1 in macrophages. Respir Res 11(1):158. https://doi.org/10.1186/1465-9921-11-158
Chen F, He Y (2009) Caspase-2 mediated apoptotic and necrotic murine macrophage cell death induced by rough Brucella abortus. PLoS ONE 4(8):e6830. https://doi.org/10.1371/journal.pone.0006830
Dong W, Zhang M, Zhu Y et al (2017) Protective effect of NSA on intestinal epithelial cells in a necroptosis model. Oncotarget 8(49):86726. https://doi.org/10.18632/oncotarget.21418
Xuan Y, Hu X (2009) Naturally-occurring shikonin analogues–a class of necroptotic inducers that circumvent cancer drug resistance. Cancer Lett 274(2):233–242. https://doi.org/10.1016/j.canlet.2008.09.029
Hu X, Han W, Li L (2007) Targeting the weak point of cancer by induction of necroptosis. Autophagy 3(5):490–492. https://doi.org/10.4161/auto.4592
Acknowledgments
This article was funded by the National Natural Science Foundation of China (Nos. 31702263, 31972715), the Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (31520103917) and the China Postdoctoral Science Foundation (No. 2017M622346).
Author information
Authors and Affiliations
Contributions
XX and JH established the main idea of the manuscript. XX and SW wrote the manuscript and prepared the figures. LL and GZ contributed with new ideas and references of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xia, X., Lei, L., Wang, S. et al. Necroptosis and its role in infectious diseases. Apoptosis 25, 169–178 (2020). https://doi.org/10.1007/s10495-019-01589-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-019-01589-x