Abstract
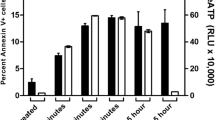
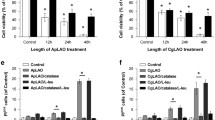
Cytolethal distending toxin (CDT) which is produced by Aggregatibacter actinomycetemcomitans causes apoptosis in lymphocytes. But the specific mechanism is not clear. The aim of our research was to investigate the effect and mechanism during this process. The wild-type CdtA, CdtB, CdtC (CdtAW, CdtBW, CdtCW) and mutant CdtB (CdtBM) were expressed and purified respectively and the purity of each subunit was examined by BandScan software. And the type I deoxyribonuclease and PI-3,4,5-triphosphate (PI-3,4,5-P3, PIP3) phosphatase activity were detected by DNA agarose gel electrophoresis and enzyme-linked immunosorbent assay respectively. The cell apoptosis rates were analyzed by flow cytometry. The morphological changes of apoptosis cells were observed by confocal laser scanning microscopy. The protein expression of Bax and Bcl-2 was examined by western blot. Differentially expressed apoptosis-related proteins were identified based on isobaric tags for relative and absolute quantitation technology. In the present study we found that: (i) recombinant wild-type CdtA, CdtB and CdtC (CdtAW, CdtBW, CdtCW) and mutant CdtB (CdtBM) were correctly expressed and the purity of each protein was higher than 80%, (ii) the average apoptosis rate in wild-type CDT (CDTW) treated groups was 50.54%, which was significantly higher than the controls (4.71%) and mutant CDT (CDTM) treated groups (5.58%) (p < 0.05), (iii) morphological changes of apoptosis were observed in CDTW treated cells, (iv) the expression of Bax protein was significantly increased in CDTW treated cells, while Bcl-2 protein expression was significantly decreased, (v) 17 apoptosis-related proteins were expressed differentially, among which 10 proteins (SMNDC1, TNFRSF10B, UBE2I, ITM2A, CASP3, P53, EIF1, TCF3, HMGN5, CASP8) were up-regulated and 7 proteins (RRM2, TPX2, KIF11, NUCKS1, TOP2A, XRCC1, PTPLAD1, RRM2) were down-regulated, (vi) one possible apoptotic pathway [Ubc9 (UBE2I)/P53/DR5 (TNFRSF10B)/Caspase-8 (CASP8)/ Caspase-3 (CASP3)] was selected and partially proved.








Similar content being viewed by others
References
Shenker BJ, Dlakic M, Walker LP, Besack D, Jaffe E, LaBelle E, Boesze-Battaglia K (2007) A novel mode of action for a microbial-derived immunotoxin: the cytolethal distending toxin subunit B exhibits phosphatidylinositol 3,4,5-triphosphate phosphatase activity. J Immunol 178:5099–5108
Li L, Ding C, Duan JL, Yang MF, Sun Y, Wang XQ, Xu Y (2013) A new functional site W115 in CdtA is critical for Aggregatibacter actinomycetemcomitans cytolethal distending toxin. PLoS ONE 8:e65729. doi:10.1371/journal.pone.0065729
Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen AM, Asikainen S, Wai SN, Oscarsson J (2012) Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect Immun 80:31–42. doi:10.1128/IAI.06069-11
Rabin SD, Flitton JG, Demuth DR (2009) Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces apoptosis in nonproliferating macrophages by a phosphatase-independent mechanism. Infect Immun 77:3161–3169. doi:10.1128/IAI.01227-08
Jankova L, Chan C, Fung CL, Song X, Kwun SY, Cowley MJ, Kaplan W, Dent OF, Bokey EL, Chapuis PH, Baker MS, Robertson GR, Clarke SJ, Molloy MP (2011) Proteomic comparison of colorectal tumours and non-neoplastic mucosa from paired patient samples using iTRAQ mass spectrometry. Mol Biosyst 7:2997–3005. doi:10.1039/c1mb05236e
Ow SY, Salim M, Noirel J, Evans C, Rehman I, Wright PC (2009) iTRAQ underestimation in simple and complex mixtures: “the good, the bad and the ugly”. J Proteome Res 8:5347–5355. doi:10.1021/pr900634c
Brenner D, Mak TW (2009) Mitochondrial cell death effectors. Curr Opin Cell Biol 21: 871–877. doi:10.1016/j.ceb.2009.09.004
Cortes-Bratti X, Frisan T, Thelestam M (2001) The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon 39: 1729–1736
Binnicker MJ, Williams RD, Apicella MA (2004) Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infect Immun 72: 6408–6417
Kang J, de Brito Bezerra B, Pacios S, Andriankaja O, Li Y, Tsiagbe V, Schreiner H, Fine DH, Graves DT (2012) Aggregatibacter actinomycetemcomitans infection enhances apoptosis in vivo through a caspase-3-dependent mechanism in experimental periodontitis. Infect Immun 80:2247–2256. doi:10.1128/IAI.06371-11
Guerra L, Cortes-Bratti X, Guidi R, Frisan T (2011) The biology of the cytolethal distending toxins. Toxins 3: 172–190. doi:10.3390/toxins3030172
Kang P, Korostoff J, Volgina A, Grzesik W, DiRienzo JM (2005) Differential effect of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans on co-cultures of human oral cells. J Med Microbiol 54: 785–794
Belibasakis GN, Brage M, Lagergard T, Johansson A (2008) Cytolethal distending toxin upregulates RANKL expression in Jurkat T-cells. Apmis 116: 499–506.
Shenker BJ, Hoffmaster RH, Zekavat A, Yamaguchi N, Lally ET, Demuth DR (2001) Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J Immunol 167:435–441
Ohara M, Hayashi T, Kusunoki Y, Miyauchi M, Takata T, Sugai M (2004) Caspase-2 and caspase-7 are involved in cytolethal distending toxin-induced apoptosis in Jurkat and MOLT-4 T-cell lines. Infect Immun 72: 871–879
Schlage P, Auf dem Keller U (2015) Proteomic approaches to uncover MMP function. Matrix Biol 44–46: 232–238 doi:10.1016/j.matbio.2015.01.003
van Raam BJ, Salvesen GS (2012) Proliferative versus apoptotic functions of caspase-8 Hetero or homo: the caspase-8 dimer controls cell fate. Biochim Biophys Acta 1:113–122. doi:10.1016/j.bbapap.2011.06.005
Ottina E, Tischner D, Herold MJ, Villunger A (2012) A1/Bfl-1 in leukocyte development and cell death. Exp Cell Res 318: 1291–1303. doi:10.1016/j.yexcr.2012.01.021
Shima H, Suzuki H, Sun J, Kono K, Shi L, Kinomura A, Ikura T, Ikura M, Kanaar R, Igarashi K, Saitoh H, Kurumizaka H, Tashiro S (2013) Activation of the SUMO modification system is required for the accumulation of RAD51 at sites of DNA damage. J Cell Sci 126: 5284–5292. doi:10.1242/jcs.133744
Kalocsay M, Hiller NJ, Jentsch S (2009) Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol Cell 33: 335–343. doi:10.1016/j.molcel.2009.01.016
Galanty Y, Belotserkovskaya R, Coates J, Polo S, Miller KM, Jackson SP (2009) Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 462: 935–939. doi:10.1038/nature08657
Morris JR, Boutell C, Keppler M, Densham R, Weekes D, Alamshah A, Galanty Y, Pangon L, Kiuchi T, Ng T, Solomon E (2009) The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 462: 886–890. doi:10.1038/nature08593
Nagai S, Davoodi N, Gasser SM (2011) Nuclear organization in genome stability: SUMO connections. Cell Res 21: 474–485. doi:10.1038/cr.2011.31
Ulrich HD (2012) Ubiquitin and SUMO in DNA repair at a glance. J Cell Sci 125: 249–254. doi:10.1242/jcs.091801
Jackson SP, Durocher D (2013) Regulation of DNA damage responses by ubiquitin and SUMO. Mol Cell 49: 795–807. doi:10.1016/j.molcel.2013.01.017
Xu-Monette ZY, Medeiros LJ, Li Y, Orlowski RZ, Andreeff M, Bueso-Ramos CE, Greiner TC, McDonnell TJ, Young KH (2012) Dysfunction of the TP53 tumor suppressor gene in lymphoid malignancies. Blood 119: 3668–3683. doi:10.1182/blood-2011-11-366062
Green DR, Kroemer G (2009) Cytoplasm functions of the tumour suppressor p53. Nature 458:1127–1130. doi:10.1038/nature07986
Vousden KH, Prives C (2009) Blinded by the Light: The Growing Complexity of p53. Cell 137:413–431. doi:10.1016/j.cell.2009.04.037
Gomes R, Guerra-Sa R, Arruda E (2010) Coxsackievirus B5 induced apoptosis of HeLa cells: effects on p53 and SUMO. Virology 396:256–263. doi:10.1016/j.virol.2009.10.005
Bennett RL, Pan Y, Christian J, Hui T, May WS Jr (2012) The RAX/PACT-PKR stress response pathway promotes p53 sumoylation and activation, leading to G(1) arrest. Cell Cycle 11: 407–417. doi:10.4161/cc.11.2.18999
Hickey TE, Majam G, Guerry P (2005) Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect Immun 73:5194–5197
Miyashita T, Reed JC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299
Lane DP (1992) Cancer. p53, guardian of the genome. Nature 358:15–16
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825
Bito T, Ueda M, Ahmed NU, Nagano T, Ichihashi M (1995) Cyclin D and retinoblastoma gene product expression in actinic keratosis and cutaneous squamous cell carcinoma in relation to p53 expression. J Cutan Pathol 22: 427–434
Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, Takei Y, Nakamura Y (2000) A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature 404:42–49
Wiezorek J, Holland P, Graves J (2010) Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res 16:1701–1708. doi:10.1158/1078-0432.CCR-09-1692
Allen JE, El-Deiry WS (2012) Regulation of the human TRAIL gene. Cancer Biol Ther 13:1143–1151. doi:10.4161/cbt.21354
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–120. doi:10.1038/cdd.2011.96
Snigdha S, Smith ED, Prieto GA, Cotman CW (2012) Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci Bull 28:14–24. doi:10.1007/s12264-012-1057-5
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81170962,81470749), Natural Science Research of University of Jiangsu Province (Grant No. 14KJD320001) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, 2014-37). We also thank American Journal Experts for revising the English used in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Hui-ping Chen, Lu Li and Xu Chen contributed equally to this study.
Rights and permissions
About this article
Cite this article
Chen, Hp., Li, L., Chen, X. et al. The mechanism of Jurkat cells apoptosis induced by Aggregatibacter actinomycetemcomitans cytolethal distending toxin. Apoptosis 22, 841–851 (2017). https://doi.org/10.1007/s10495-017-1357-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1357-3