Abstract
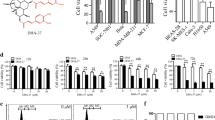
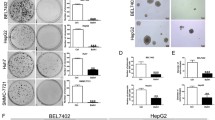
This report is designed to dissect the detail molecular mechanism by which dihydroartemisinin (DHA), a derivative of artemisinin, induces apoptosis in human hepatocellular carcinoma (HCC) cells. DHA induced a loss of the mitochondrial transmemberane potential (ΔΨm), release of cytochrome c, activation of caspases, and externalization of phosphatidylserine indicative of apoptosis induction. Compared with the modest inhibitory effects of silencing Bax, silencing Bak largely prevented DHA-induced ΔΨm collapse and apoptosis though DHA induced a commensurable activation of Bax and Bak, demonstrating a key role of the Bak-mediated intrinsic apoptosis pathway. DHA did not induce Bid cleavage and translocation from cytoplasm to mitochondria and had little effects on the expressions of Puma and Noxa, but did increase Bim and Bak expressions and decrease Mcl-1 expression. Furthermore, the cytotoxicity of DHA was remarkably reduced by silencing Bim, and modestly but significantly reduced by silencing Puma or Noxa. Silencing Bim or Noxa preferentially reduced DHA-induced Bak activation, while silencing Puma preferentially reduced DHA-induced Bax activation, demonstrating that Bim and to a lesser extent Noxa act as upstream mediators to trigger the Bak-mediated intrinsic apoptosis pathway. In addition, silencing Mcl-1 enhanced DHA-induced Bak activation and apoptosis. Taken together, our data demonstrate a crucial role of Bim in preferentially regulating the Bak/Mcl-1 rheostat to mediate DHA-induced apoptosis in HCC cells.







Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
El-Serag HB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142(6):1264–1273
Bruix J, Gores GJ, Mazzaferro V (2014) Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 63(5):844–855
Yang JD, Roberts LR (2010) Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol 7(8):448–458
Marquardt JU, Thorgeirsson SS (2014) SnapShot: hepatocellular carcinoma. Cancer Cell 25(4):550
Camma C, Cabibbo G, Petta S, Enea M, Iavarone M, Grieco A et al (2013) Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology 57(3):1046–1054
Abdel-Rahman O, Fouad M (2014) Sorafenib-based combination as a first line treatment for advanced hepatocellular carcinoma: a systematic review of the literature. Crit Rev Oncol Hematol 91(1):1–8
Avila MA, Berasain C, Sangro B, Prieto J (2006) New therapies for hepatocellular carcinoma. Oncogene 25(27):3866–3884
Klein J, Dawson LA (2013) Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 87(1):22–32
Chaturvedi D, Goswami A, Saikia PP, Barua NC, Rao PG (2010) Artemisinin and its derivatives: a novel class of anti-malarial and anti-cancer agents. Chem Soc Rev 39(2):435–454
Ho WE, Peh HY, Chan TK, Wong WS (2014) Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther 142(1):126–139
Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B (2014) Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol Cancer Ther 10(12):2224–2233
Zhao CB, Gao WJ, Chen TS (2014) Synergistic induction of apoptosis in A549 cells by dihydroartemisinin and gemcitabine. Apoptosis 19(4):668–681
Lai HC, Singh NP, Sasaki T (2013) Development of artemisinin compounds for cancer treatment. Invest New Drugs 31(1):230–246
Hou J, Wang D, Zhang R, Wang H (2008) Experimental therapy of hepatoma with artemisinin and its derivatives: in vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res 14(17):5519–5530
Crespo-Ortiz MP, Wei MQ (2012) Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol 2012:247597
Firestone GL, Sundar SN (2009) Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med 11:e32
Wang SJ, Gao Y, Chen H, Kong R, Jiang HC, Pan SH et al (2010) Dihydroartemisinin inactivates NF-kappaB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both in vitro and in vivo. Cancer Lett 293(1):99–108
Chen T, Li M, Zhang R, Wang H (2009) Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J Cell Mol Med 13(7):1358–1370
Zhao CB, Qin GQ, Gao WJ, Chen JQ, Liu HY, Xi GN et al (2014) Potent proapoptotic actions of dihydroartemisinin in gemcitabine-resistant A549 cells. Cell Signal 26(10):2223–2233
Ji Y, Zhang YC, Pei LB, Shi LL, Yan JL, Ma XH (2011) Anti-tumor effects of dihydroartemisinin on human osteosarcoma. Mol Cell Biochem 351(1–2):99–108
Sun H, Meng X, Han J, Zhang Z, Wang B, Bai X, Zhang X (2013) Anti-cancer activity of DHA on gastric cancer–an in vitro and in vivo study. Tumour Biol 34(6):3791–3800
Lu YY, Chen TS, Wang XP, Qu JL, Chen M (2010) The JNK inhibitor SP600125 enhances dihydroartemisinin-induced apoptosis by accelerating Bax translocation into mitochondria in human lung adenocarcinoma cells. FEBS Lett 584(18):4019–4026
Chen TS, Chen M, Chen JQ (2013) Ionizing radiation potentiates dihydroartemisinin-induced apoptosis of A549 cells via a caspase-8-dependent pathway. PLoS ONE 8(3):e59827
Handrick R, Ontikatze T, Bauer KD, Freier F, Rubel A, Durig J et al (2010) Dihydroartemisinin induces apoptosis by a Bak-dependent intrinsic pathway. Mol Cancer Ther 9(9):2497–2510
Gao XL, Luo Z, Xiang TX, Wang KJ, Li J, Wang PL (2011) Dihydroartemisinin induces endoplasmic reticulum stress-mediated apoptosis in HepG2 human hepatoma cells. Tumori 97(6):771–780
Zhang CZ, Zhang H, Yun J, Chen GG, Lai PB (2012) Dihydroartemisinin exhibits antitumor activity toward hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol 83(9):1278–1289
Zhang CZ, Pan Y, Cao Y, Lai PB, Liu L, Chen GG, Yun J (2012) Histone deacetylase inhibitors facilitate dihydroartemisinin-induced apoptosis in liver cancer in vitro and in vivo. PLoS ONE 7(6):e39870
Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP et al (2009) Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 36(3):487–499
Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL et al (2011) A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell 44(4):517–531
Zhou CJ, Pan WL, Wang XP, Chen TS (2012) Artesunate induces apoptosis via a Bak-mediated caspase-independent intrinsic pathway in human lung adenocarcinoma cells. J Cell Physiol 227(12):3778–3786
Mao H, Gu H, Qu X, Sun J, Song B, Gao W et al (2013) Involvement of the mitochondrial pathway and Bim/Bcl-2 balance in dihydroartemisinin-induced apoptosis in human breast cancer in vitro. Int J Mol Med 31(1):213–218
Ontikatze T, Rudner J, Handrick R, Belka C, Jendrossek V (2014) Dihydroartemisinin is a hypoxia-active anti-cancer drug in colorectal carcinoma cells. Front Oncol 4:116
Cabello CM, Lamore SD, Bair WB III, Qiao S, Azimian S, Lesson JL, Wondrak GT (2012) The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis. Invest New Drugs 30(4):1289–1301
Gao WJ, Xiao FL, Wang XP, Chen TS (2013) Artemisinin induces A549 cell apoptosis dominantly via a reactive oxygen species-mediated amplification activation loop among caspase-9, -8 and -3. Apoptosis 18(10):1201–1213
Liang Q, Wang XP, Chen TS (2014) Resveratrol protects rabbit articular chondrocyte against sodium nitroprusside-induced apoptosis via scavenging ROS. Apoptosis 19(9):1354–1363
Lu YY, Chen TS, Wang XP, Li L (2010) Single-cell analysis of dihydroartemisinin-induced apoptosis through reactive oxygen species-mediated caspase-8 activation and mitochondrial pathway in ASTC-a-1 cells using fluorescence imaging techniques. J Biomed Opt 15(4):046028
Koyama T, Kume S, Koya D, Araki S, Isshiki K, Chin-Kanasaki M et al (2011) SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radic Biol Med 51(6):1258–1267
Onuki R, Nagasaki A, Kawasaki H, Baba T, Uyeda TQ, Taira K (2002) Confirmation by FRET in individual living cells of the absence of significant amyloid beta -mediated caspase 8 activation. Proc Natl Acad Sci USA 99(23):14716–14721
Takikawa Y, Miyoshi H, Rust C, Roberts P, Siegel R, Mandal PK et al (2001) The bile acid-activated phosphatidylinositol 3-kinase pathway inhibits Fas apoptosis upstream of bid in rodent hepatocytes. Gastroenterology 120(7):1810–1817
Chen S, Dai Y, Harada H, Dent P, Grant S (2007) Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res 67(2):782–791
Zhang WW, Wang XP, Chen TS (2012) Resveratrol induces apoptosis via a Bak-mediated intrinsic pathway in human lung adenocarcinoma cells. Cell Signal 24(5):1037–1046
McDonnell MA, Wang D, Khan SM, Vander Heiden MG, Kelekar A (2003) Caspase-9 is activated in a cytochrome c-independent manner early during TNFα-induced apoptosis in murine cells. Cell Death Differ 10(9):1005–1015
Plotz M, Gillissen B, Hossini AM, Daniel PT, Eberle J (2012) Disruption of the VDAC2-Bak interaction by Bcl-x(S) mediates efficient induction of apoptosis in melanoma cells. Cell Death Differ 19(12):1928–1938
Rehman K, Tariq M, Akash MS, Gillani Z, Qazi MH (2014) Effect of HA14-1 on apoptosis-regulating proteins in HeLa cells. Chem Biol Drug Des 83(3):317–323
Neise D, Graupner V, Gillissen BF, Daniel PT, Schulze-Osthoff K, Janicke RU, Essmann F (2008) Activation of the mitochondrial death pathway is commonly mediated by a preferential engagement of Bak. Oncogene 27(10):1387–1396
Ba Q, Zhou N, Duan J, Chen T, Hao M, Yang X et al (2012) Dihydroartemisinin exerts its anticancer activity through depleting cellular iron via transferrin receptor-1. PLoS ONE 7(8):e42703
Lu JJ, Yang Z, Lu DZ, Wo XD, Shi JJ, Lin TQ et al (2012) Dihydroartemisinin-induced inhibition of proliferation in BEL-7402 cells: an analysis of the mitochondrial proteome. Mol Med Rep 6(2):429–433
Kutuk O, Arisan ED, Tezil T, Shoshan MC, Basaga H (2009) Cisplatin overcomes Bcl-2-mediated resistance to apoptosis via preferential engagement of Bak: critical role of Noxa-mediated lipid peroxidation. Carcinogenesis 30(9):1517–1527
Prochazka L, Dong LF, Valis K, Freeman R, Ralph SJ, Turanek J, Neuzil J (2010) alpha-Tocopheryl succinate causes mitochondrial permeabilization by preferential formation of Bak channels. Apoptosis 15(7):782–794
Karlberg M, Ekoff M, Labi V, Strasser A, Huang D, Nilsson G (2010) Pro-apoptotic Bax is the major and Bak an auxiliary effector in cytokine deprivation-induced mast cell apoptosis. Cell Death Dis 1:e43
Kim BJ, Ryu SW, Song BJ (2006) JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem 281(30):21256–21265
Simonin K, Brotin E, Dufort S, Dutoit S, Goux D, N’Diaye M et al (2009) Mcl-1 is an important determinant of the apoptotic response to the BH3-mimetic molecule HA14-1 in cisplatin-resistant ovarian carcinoma cells. Mol Cancer Ther 8(11):3162–3170
Jiao Y, Tong J (2007) Dihydroartemisinin is an inhibitor of ovarian cancer cell growth1. Acta Pharmacol Sin 28(7):1045–1056
Gao N, Budhraja A, Cheng S, Liu EH, Huang C, Chen J et al (2011) Interruption of the MEK/ERK signaling cascade promotes dihydroartemisinin-induced apoptosis in vitro and in vivo. Apoptosis 16(5):511–523
Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI et al (2005) Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19(11):1294–1305
Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S et al (2006) Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol 44(1):151–157
Chen M, Chen TS, Lu YY, Liu CY, Qu JL (2012) Dihydroarteminsin-induced apoptosis is not dependent on the translocation of Bim to the endoplasmic reticulum in human lung adenocarcinoma cells. Pathol Oncol Res 18(4):809–816
Huang S, Sinicrope FA (2008) BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res 68(8):2944–2951
Michalak EM, Villunger A, Adams JM, Strasser A (2008) In several cell types tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ 15(6):1019–1029
Han J, Goldstein LA, Hou W, Rabinowich H (2007) Functional linkage between NOXA and Bim in mitochondrial apoptotic events. J Biol Chem 282(22):16223–16231
Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G et al (2006) Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med 203(13):2939–2951
Acknowledgments
The authors thank Dr. K. Taira for providing CFP-Bid plasmid and Dr. G. J. Gores for providing GFP Cyt.c plasmid. This work was supported by National Natural Science Foundation of China (Nos. 61178078, 81471699 and 61308111) and Guangzhou science and technology plan project (No. 2014J4100055).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qin, G., Zhao, C., Zhang, L. et al. Dihydroartemisinin induces apoptosis preferentially via a Bim-mediated intrinsic pathway in hepatocarcinoma cells. Apoptosis 20, 1072–1086 (2015). https://doi.org/10.1007/s10495-015-1132-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1132-2