Abstract
Mammalian target of rapamycin (mTOR) is a central kinase that regulates cell survival, proliferation and translation. Reactive oxygen species (ROS) are second messengers with potential in manipulating cellular signaling. Here we report that two ROS generating phytochemicals, hydroxychavicol and curcumin synergize in leukemic cells in inducing enhanced apoptosis by independently activating both mitogen activated protein kinase (MAPK) (JNK and P38) and mTOR pathways. Low level transient ROS generated after co-treatment with these phytochemicals led to activation of these two pathways. Both mTOR and MAPK pathways played important roles in co-treatment-induced apoptosis, by knocking down either mTOR or MAPKs inhibited apoptosis. Activation of mTOR, as evident from phosphorylation of its downstream effector eukaryotic translation initiation factor 4E-binding protein 1, led to release of eukaryotic translation initiation factor 4E (eIF4E) which was subsequently phosphorylated by JNK leading to translation of pro-apoptotic proteins Bax and Bad without affecting the expression of anti-apoptotic protein Bcl-xl. Our data suggest that mTOR and MAPK pathways converge at eIF4E in co-treatment-induced enhanced apoptosis and provide mechanistic insight for the role of mTOR activation in apoptosis.







Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- JNK:
-
c-Jun-N-terminal kinase
- FITC:
-
Fluorescein isothiocyanate
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- mTOR:
-
Mammalian target of rapamycin
- MAPK:
-
Mitogen activated protein kinase
- eIF4E:
-
Eukaryotic translation initiation factor 4E
- 4E-BP1:
-
Eukaryotic translation initiation factor 4E-binding protein 1
References
Wang J, Yi J (2008) Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther 7:1875–1884
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P (2005) Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol 25:6391–6403
Zündorf G, Reiser G (2011) Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal 14:1275–1288
Malik F, Kumar A, Bhushan S, Khan S, Bhatia A, Suri KA, Qazi GN, Singh J (2007) Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis 12:2115–2133
Wada T, Penninger JM (2004) Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23:2349–2838
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293
Ishikawa D, Takeuchi S, Nakagawa T, Sano T, Nakade J, Nanjo S, Yamada T, Ebi H, Zhao L, Yasumoto K, Nakamura T, Matsumoto K, Kagamu H, Yoshizawa H, Yano S (2013) mTOR inhibitors control the growth of EGFR mutant lung cancer even after acquiring resistance by HGF. PLoS ONE 8:e62104
Vignot S, Faivre S, Aguirre D, Raymond E (2005) mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol 16:525–537
Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen W, Shen T, Han X, Kontos CD, Huang S (2011) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic Biol Med 50:624–632
Kato H, Nakajima S, Saito Y, Takahashi S, Katoh R, Kitamura M (2012) mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell Death Differ 19:310–320
Xu B, Chen S, Luo Y, Chen Z, Liu L, Zhou H, Chen W, Shen T, Han X, Chen L, Huang S (2011) Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS ONE 6:e19052
Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N (1996) Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J 15:658–664
Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe SW (2007) Dissecting eIF4E action in tumorigenesis. Genes Dev 21:3232–3237
Jiang Y, Zhang SH, Han GQ, Qin CY (2010) Interaction of Pdcd4 with eIF4E inhibits the metastatic potential of hepatocellular carcinoma. Biomed Pharmacother 64:424–429
Shveygert M, Kaiser C, Bradrick SS, Gromeier M (2010) Regulation of eukaryotic initiation factor 4E (eIF4E) phosphorylation by mitogen-activated protein kinase occurs through modulation of Mnk1–eIF4G interaction. Mol Cell Biol 21:5160–5167
Duncan RF, Peterson H, Sevanian A (2005) Signal transduction pathways leading to increased eIF4E phosphorylation caused by oxidative stress. Free Radic Biol Med 38:631–643
Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, Petroulakis E, Robichaud N, Pollak M, Gaboury LA, Pandolfi PP, Saad F, Sonenberg N (2010) eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA 107:14134–14139
Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL (2005) eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. J Cell Biol 169:245–256
Li S, Takasu T, Perlman DM, Peterson MS, Burrichter D, Avdulov S, Bitterman PB, Polunovsky VA (2003) Translation factor eIF4E rescues cells from Myc-dependent apoptosis by inhibiting cytochrome c release. J Biol Chem 278:3015–3022
Chakraborty JB, Mahato SK, Joshi K, Shinde V, Rakshit S, Biswas N, Mukherjee IC, Mandal L, Ganguly D, Chowdhury AA, Chaudhuri J, Paul K, Pal BC, Vinayagam J, Pal C, Manna A, Jaisankar P, Chaudhuri U, Konar A, Roy S, Bandyopadhyay S (2012) Hydroxychavicol, a Piper betel leaf component, induces apoptosis of CML cells through mitochondrial reactive oxygen species-dependent JNK and endothelial nitric oxide synthase activation and overrides imatinib resistance. Cancer Sci 103:88–99
Yu J, Peng Y, Wu LC, Xie Z, Deng Y, Hughes T, He S, Mo X, Chiu M, Wang QE, He X, Liu S, Grever MR, Chan KK, Liu Z (2013) Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukemic role in acute myeloid leukemia. PLoS ONE 8:e55934
Lozzio CB, Lozzio BB (1975) Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45:321–334
Bandyopadhyay G, Biswas T, Roy KC, Mandal S, Mandal C, Pal BC, Bhattacharya S, Rakshit S, Bhattacharya DK, Chaudhuri U, Konar A, Bandyopadhyay S (2004) Chlorogenic acid inhibits Bcr-Abl tyrosine kinase and triggers p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemic cells. Blood 104:2514–2522
Kubonishi I, Miyoshi I (1983) Establishment of a Ph1 chromosome-positive cell line from chronic myelogenous leukemia in blast crisis. Int J Cell Cloning 1:105–117
Friis MB, Friborg CR, Schneider L, Nielsen MB, Lambert IH, Christensen ST, Hoffmann EK (2005) Cell shrinkage as a signal to apoptosis in NIH 3T3 fibroblasts. J Physiol 567:427–443
Ravindra PV, Tiwari AK, Ratta B, Chaturvedi U, Palia SK, Subudhi PK, Kumar R, Sharma B, Rai A, Chauhan RS (2008) Induction of apoptosis in Vero cells by Newcastle disease virus requires viral replication, de novo protein synthesis and caspase activation. Virus Res 133:285–290
Rakshit S, Mandal L, Pal BC, Bagchi J, Biswas N, Chaudhuri J, Chowdhury AA, Manna A, Chaudhuri U, Konar A, Mukherjee T, Jaisankar P, Bandyopadhyay S (2010) Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl + CML cells. Biochem Pharmacol 80:1662–1675
Bharti AC, Donato N, Singh S, Aggarwal BB (2003) Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 10:1053–1062
Berenbaum MC (1981) Criteria for analyzing interactions between biologically active agents. Adv Cancer Res 35:269–335
Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, Huang S (2009) Curcumin disrupts the mammalian target of rapamycin–raptor complex. Cancer Res 69:1000–1008
Chou TC, Talalay P (1984) Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul 22:27–55
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030
Lee YJ, Kim NY, Suh YA, Lee C (2011) Involvement of ROS in curcumin-induced autophagic cell death. Korean J Physiol Pharmacol 15:1–7
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8:579–591
Wang WZ, Li L, Liu MY, Jin XB, Mao JW, Pu QH, Meng MJ, Chen XG, Zhu JY (2013) Curcumin induces FasL-related apoptosis through p38 activation in human hepatocellular carcinoma Huh7 cells. Life Sci 92:352–358
Chen MB, Zhang Y, Wei MX, Shen W, Wu XY, Yao C, Lu PH (2013) Activation of AMP-activated protein kinase (AMPK) mediates plumbagin-induced apoptosis and growth inhibition in cultured human colon cancer cells. Cell Signal 25:1993–2002
Moreno-Manzano V, Ishikawa Y, Lucio-Cazana J, Kitamura M (2000) Selective involvement of superoxide anion, but not downstream compounds hydrogen peroxide and peroxynitrite, in tumor necrosis factor-alpha-induced apoptosis of rat mesangial cells. J Biol Chem 275:12684–12691
O’Brien PJ (1991) Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact 80:1–41
Sakano K, Inagaki Y, Oikawa S, Hiraku Y, Kawanishi S (2004) Copper-mediated oxidative DNA damage induced by eugenol: possible involvement of O-demethylation. Mutat Res 565:35–44
Fang J, Lu J, Holmgren A (2005) Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem 280:25284–25290
Atsumi T, Fujisawa S, Tonosaki K (2005) Relationship between intracellular ROS production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis 11:236–242
Silva A, Gírio A, Cebola I, Santos CI, Antunes F, Barata JT (2011) Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia 25:960–967
Su X, Wang P, Wang X, Guo L, Li S, Liu Q (2013) Involvement of MAPK activation and ROS generation in human leukemia U937 cells undergoing apoptosis in response to sonodynamic therapy. Int J Radiat Biol. doi:10.3109/09553002.2013.817700
Das F, Ghosh-Choudhury N, Bera A, Kasinath BS, Choudhury GG (2013) TGFβ-induced PI 3 kinase-dependent Mnk-1 activation is necessary for Ser-209 phosphorylation of eIF4E and mesangial cell hypertrophy. J Cell Physiol. doi:10.1002/jcp.24327
Graff JR, Konicek BW, Carter JH, Marcusson EG (2008) Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res 68:631–634
Chen YJ, Tan BC, Cheng YY, Chen JS, Lee SC (2010) Differential regulation of CHOP translation by phosphorylated eIF4E under stress conditions. Nucl Acids Res 38:764–777
Tsukano H, Gotoh T, Endo M, Miyata K, Tazume H, Kadomatsu T, Yano M, Iwawaki T, Kohno K, Araki K, Mizuta H, Oike Y (2010) The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol 30:1925–1932
Acknowledgments
We thank Dr. Carlo Gambacorti-Passerini (Instituto Nazionale Tumori, Milan, Italy) for providing KCL22 cell line. We are also thankful to Mr. Surjendu Bikash Debnath for technical assistance and Dr. Anupam Banerjee for confocal microscopy. This work is financially supported by Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
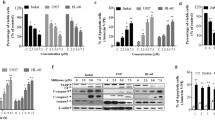
a Viability of normal cell lines were assessed by MTT after indicated treatment for 48 h. b Viability of PBMC isolated from one normal donor and two CML patients was determined by Trypan blue dye exclusion. **p < 0.01. (TIFF 8448 kb)
Fig. 2
a Bar diagram representation of annexin V positive cells of Fig. 1C, 1D and 1E. Data represent mean ± SD of three experiments. (TIFF 2565 kb)
Fig. 3
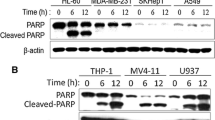
a K562 cells were treated with graded concentrations of HCH as indicated and were subjected to MTT assay for determination of viability. *p < 0.01, **p < 0.001. b Whole cell lysates of K562 cells treated with varying concentrations of HCH alone were subjected to immunoblotting. (TIFF 8440 kb)
Fig. 4
Intracellular flow cytometry analysis of pro-apoptotic and anti-apoptotic proteins in K562 cells which were transfected with mTOR siRNA followed by incubation with HCH and Cur simultaneously. Open histograms represent staining with isotype matched control antibodies. Filled histograms represent staining with specific antibodies. Values in the boxes represent specific mean fluorescence intensity after subtracting control values. (TIFF 8439 kb)
Fig. 5
a K562 cells were pretreated with NAC (5 mM) and MnTBAP (100 μM) for 1 h before treatment with HCH plus Cur in combination. MitoSOX™ Red fluorescence was then measured by flow cytometry 30 min after co-treatment. b K562 cells were fractionated into mitochondrial and cytosolic fractions after indicated treatments. Fractions were immunoblotted for cytochrom c. (TIFF 8440 kb)
Fig. 6
Intracellular flow cytometry analysis for pro-apoptotic and anti-apoptotic proteins in K562 cells after transfection with eIF4E siRNA followed by treatment with HCH plus Cur. (TIFF 328 kb)
Rights and permissions
About this article
Cite this article
Chaudhuri, J., Chowdhury, A.A., Biswas, N. et al. Superoxide activates mTOR–eIF4E–Bax route to induce enhanced apoptosis in leukemic cells. Apoptosis 19, 135–148 (2014). https://doi.org/10.1007/s10495-013-0904-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0904-9