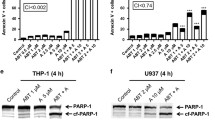
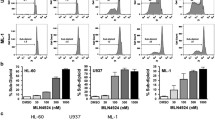
Abstract
PP2A activator FTY720 has been shown to possess the anti-leukemic activity for chronic myelogenous leukemia (CML), however, the cell killing mechanism underlying its anti-leukemic activity has remained to be verified. We investigated the precise mechanisms underlying the apoptosis induction by FTY720, especially focusing on the roles of BH3-only proteins, and the therapeutic potency of FTY720 for CML. Enforced expression of either BCL2 or the dominant-negative protein of FADD (FADD.DN) partly protected CML cells from apoptosis by FTY720, indicating the involvement of both cell extrinsic and intrinsic apoptosis pathways. FTY720 activates pro-apoptotic BH3-only proteins: BIM, which is essential for apoptosis by BCR-ABL1 tyrosine kinase inhibitors (TKIs), and BID, which accelerates the extrinsic apoptosis pathway. Gene knockdown of either BIM or BID partly protected K562 cells from apoptosis by FTY720, but the extent of cell protection was not as much as that by overexpression of either BCL2 or FADD.DN. Moreover, knockdown of both BIM and BID did not provide additional protection compared with knockdown of only BIM or BID, indicating that BIM and BID complement each other in apoptosis by FTY720, especially when either is functionally impaired. FTY720 can overcome TKI resistance caused by ABL kinase domain mutations, dysfunction of BIM resulting from gene deletion polymorphism, and galectin-3 overexpression. In addition, ABT-263, a BH3-mimetic, significantly augmented cell death induction by FTY720 both in TKI-sensitive and -resistant leukemic cells. These results provide the rationale that FTY720, with its unique effects on BIM and BID, could lead to new therapeutic strategies for CML.







Similar content being viewed by others
References
O’Brien SG, Guilhot F, Larson RA et al (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348:994–1004
Kantarjian H, Shah NP, Hochhaus A et al (2010) Dasatinib versus imatinib in newly diagnosed chronicphase chronic myeloid leukemia. N Engl J Med 362:2260–2270
Saglio G, Kim DW, Issaragrisil S et al (2010) Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 362:2251–2259
Kuroda J, Shimura Y, Yamamoto-Sugitani M, Sasaki N, Taniwaki M (2013) Multifaceted mechanisms for cell survival and drug targeting in chronic myelogenous leukemia. Curr Cancer Drug Targ 13:69–79
Kuroda J, Puthalakath H, Cragg MS et al (2006) Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA 103:14907–14912
Kuroda J, Kimura S, Strasser A et al (2007) Apoptosis-based dual molecular targeting by INNO-406, a second-generation Bcr-Abl inhibitor, and ABT-737, an inhibitor of antiapoptotic Bcl-2 proteins, against Bcr-Abl-positive leukemia. Cell Death Differ 14:1667–1677
Skaggs BJ, Gorre ME, Ryvkin A et al (2006) Phosphorylation of the ATP-binding loop directs oncogenicity of drug-resistant BCR-ABL mutants. Proc Natl Acad Sci USA 103:19466–19471
Gorre ME, Mohammed M, Ellwood K et al (2001) Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293:876–880
Yamamoto-Sugitani M, Kuroda J, Ashihara E et al (2011) Galectin-3 (Gal-3) induced by leukemia microenvironment promotes drug resistance and bone marrow lodgment in chronic myelogenous leukemia. Proc Natl Acad Sci USA 108:17468–17473
Kamitsuji Y, Kuroda J, Kimura S et al (2008) The Bcr-Abl kinase inhibitor INNO-406 induces autophagy and different modes of cell death execution in Bcr-Abl-positive leukemias. Cell Death Differ 15:1712–1722
Bellodi C, Lidonnici MR, Hamilton A et al (2009) Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest 119:1109–1123
Crowley LC, Elzinga BM, O’Sullivan GC, McKenna SL (2011) Autophagy induction by Bcr-Abl-expressing cells facilitates their recovery from a targeted or nontargeted treatment. Am J Hematol 86:38–47
Jørgensen HG, Holyoake TL (2007) Characterization of cancer stem cells in chronic myeloid leukaemia. Biochem Soc Trans 35:1347–1351
Jamieson CH, Ailles LE, Dylla SJ et al (2004) Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med 351:657–667
Lemoli RM, Salvestrini V, Bianchi E et al (2009) Molecular and functional analysis of the stem cell compartment of chronic myelogenous leukemia reveals the presence of a CD34- cell population with intrinsic resistance to imatinib. Blood 114:5191–5200
Copland M, Hamilton A, Elrick LJ et al (2006) Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood 107:4532–4539
Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ (2011) Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest 121:396–409
Takeuchi M, Kimura S, Kuroda J et al (2010) Glyoxalase-I is a novel target against Bcr-Abl+ leukemic cells acquiring stem-like characteristics in a hypoxic environment. Cell Death Differ 17:1211–1220
Schmidt T, Kharabi Masouleh B, Loges S et al (2011) Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1(+) leukemia. Cancer Cell 19:740–753
Damiano JS, Hazlehurst LA, Dalton WS (2001) Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia 15:1232–1239
Jin L, Tabe Y, Konoplev S et al (2008) CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Mol Cancer Ther 7:48–58
Wang Y, Cai D, Brendel C et al (2007) Adaptive secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) mediates imatinib and nilotinib resistance in BCR/ABL+ progenitors via JAK-2/STAT-5 pathway activation. Blood 109:2147–2155
Ng KP, Hillmer AM, Chuah CT et al (2012) A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med 18:521–528
Lucas CM, Harris RJ, Giannoudis A, Copland M, Slupsky JR, Clark RE (2011) Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood 117:6660–6668
Mahon FX, Réa D, Guilhot J et al (2010) Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre stop imatinib (STIM) trial. Lancet Oncol 11:1029–1035
Ross DM, Branford S, Seymour JF et al (2010) Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia 24:1719–1724
Stamatović D, Balint B, Tukić L et al (2012) Allogeneic stem cell transplant for chronic myeloid leukemia as a still promising option in the era of the new target therapy. Vojnosanit Pregl 69:37–42
Janssens V, Goris J (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353:417–439
Janssens V, Goris J, Van Hoof C (2005) PP2A: the expected tumor suppressor. Curr Opin Genet Dev 15:34–41
Azuma H, Takahara S, Ichimaru N et al (2002) Marked Prevention of Tumor Growth and Metastasis by a Novel Immunosuppressive Agent, FTY720, in Mouse Breast Cancer Models. Cancer Res 62:1410–1419
Calin GA, di Iasio MG, Caprini E et al (2000) Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene 19:1191–1195
Suzuki K, Takahashi K (2003) Reduced expression of the regulatory A subunit of serine/threonine protein phosphatase 2A in human breast cancer MCF-7 cells. Int J Oncol 23:1263–1268
Takagi Y, Futamura M, Yamaguchi K, Aoki S, Takahashi T, Saji S (2000) Alterations of the PPP2R1B gene located at 11q23 in human colorectal cancers. Gut 47:268–271
Neviani P, Santhanam R, Trotta R et al (2005) The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 8:355–368
Kappos L, Antel J, Comi G et al (2006) Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 355:1124–1140
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Devonshire V, Havrdova E, Radue EW et al (2012) Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol 11:420–428
Brinkmann V, Davis MD, Heise CE et al (2002) The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277:21453–21457
Mandala S, Hajdu R, Bergstrom J et al (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296:346–349
Matloubian M, Lo CG, Cinamon G et al (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427:355–360
Neviani P, Santhanam R, Oaks JJ et al (2007) FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest 117:2408–2421
Liao A, Broeg K, Fox T et al (2011) Therapeutic efficacy of FTY720 in a rat model of NK-cell leukemia. Blood 118:2793–2800
Liu Q, Zhao X, Frissora F et al (2008) FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood 111:275–284
Liu Q, Alinari L, Chen CS et al (2010) FTY720 shows promising in vitro and in vivo preclinical activity by downmodulating Cyclin D1 and phospho-Akt in mantle cell lymphoma. Clin Cancer Res 16:3182–3192
Roberts KG, Smith AM, McDougall F et al (2010) Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers. Cancer Res 70:5438–5447
Shinomiya T, Li XK, Amemiya H, Suzuki S (1997) An immunosuppressive agent, FTY720, increases intracellular concentration of calcium ion and induces apoptosis in HL-60. Immunology 91:594–600
Permpongkosol S, Wang JD, Takahara S et al (2002) Anticarcinogenic effect of FTY720 in human prostate carcinoma DU145 cells: modulation of mitogenic signaling, FAK, cell-cycle entry and apoptosis. Int J Cancer 98:167–172
Brinkmann V, Wilt C, Kristofic C et al (2001) FTY720: dissection of membrane receptor-operated, stereospecific effects on cell migration from receptor-independent antiproliferative and apoptotic effects. Transplant Proc 33:3078–3080
Ogretmen B, Hannun YA (2004) Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 4:604–616
Suzuki E, Handa K, Toledo MS, Hakomori S (2004) Sphingosine-dependent apoptosis: a unified concept based on multiple mechanisms operating in concert. Proc Natl Acad Sci USA 101:14788–14793
Shimura Y, Kuroda J, Ri M et al (2012) RSK2Ser227 at N-terminal kinase domain is a potential therapeutic target for multiple myeloma. Mol Cancer Ther 11:2600–2609
Ricci C, Scappini B, Divoky V et al (2002) Mutation in the ATP-binding pocket of the ABL kinase domain in an STI571-resistant BCR/ABL-positive cell line. Cancer Res 62:5995–5998
Kuroda J, Taniwaki M (2009) Involvement of BH3-only proteins in hematologic malignancies. Crit Rev Oncol Hematol 71:89–101
Kuwana T, Bouchier-Hayes L, Chipuk JE et al (2005) BH3 domeins of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17:525–535
Willis SN, Fletcher JI, Kaufmann T et al (2007) Apopotosis initiated when BH3 ligands engage multiple Bcl-2 homologys, not Bax or Bak. Science 315:856–859
Kuroda J, Kimura S, Andreeff M et al (2008) ABT-737 is a useful component of combinatory chemotherapies for chronic myeloid leukaemias with diverse drug-resistance mechanisms. Br J Haematol 140:181–190
Burke BA, Carroll M (2010) BCR-ABL: a multi-faceted promoter of DNA mutation in chronic myelogeneous leukemia. Leukemia 24:1105–1112
San José-Eneriz E, Agirre X, Jiménez-Velasco A et al (2009) Epigenetic down-regulation of BIM expression is associated with reduced optimal responses to imatinib treatment in chronic myeloid leukaemia. Eur J Cancer 45:1877–1889
Kuroda J, Yamamoto M, Nagoshi H et al (2010) Targeting activating transcription factor 3 by Galectin-9 induces apoptosis and overcomes various types of treatment resistance in chronic myelogenous leukemia. Mol Cancer Res 8:994–1001
Česen MH, Pegan K, Spes A, Turk B (2012) Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res 318:1245–1251
Acknowledgments
This study was partly supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Masafumi Taniwaki, Junya Kuroda and Mio Yamamoto-Sugitani), the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the KANAE Foundation for the Promotion of Medical Science, the Hoansha Foundation and the Award in Aki’s Memory from the International Myeloma Foundation (Junya Kuroda).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1
Effects of FTY720 on BAD, NOXA and PUMA. K562 cells were treated with either 7.5 M FTY720 or 0.5 M IM for the indicated periods. No clear activation or induction was observed in BAD, NOXA or PUMA by FTY720 treatment. Positive controls (P) of NOXA and PUMA are also shown with molecular marker (M) (TIF 139 kb)
Rights and permissions
About this article
Cite this article
Kiyota, M., Kuroda, J., Yamamoto-Sugitani, M. et al. FTY720 induces apoptosis of chronic myelogenous leukemia cells via dual activation of BIM and BID and overcomes various types of resistance to tyrosine kinase inhibitors. Apoptosis 18, 1437–1446 (2013). https://doi.org/10.1007/s10495-013-0882-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0882-y