Abstract
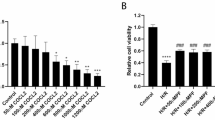
Reactive oxygen species (ROS) contribute significantly to apoptosis in renal ischemia-reperfusion (IR) injury, however the exact mechanisms are not well understood. We used novel lentiviral vectors to over-express superoxide dismutase 1 (SOD1) in proximal tubular epithelial (LLC-PK1) cells and determined effects of SOD1 following ATP depletion-recovery, used as a model to simulate renal IR. SOD1 over-expression partially protected against cytotoxicity (P < 0.001) and decreased superoxide (O2 •−) in ATP depleted cells. The ATP depletion-mediated increase in nuclear fragmentation, an index of apoptosis and activation of caspase-3 was also partially blocked by SOD1 (P < 0.05). However, SOD1 over-expression was insufficient to completely attenuate caspase-3, indicating that ROS other than cytoplasmic O2 •− are involved in ATP depletion mediated injury. To test the contribution of hydrogen peroxide, a subset of enhanced green fluorescent protein (EGFP) and SOD1 (serum free and injured) cells were treated with polyethylene glycol-catalase (PEG-catalase). As expected there was 50% reduction in cytotoxicity and caspase-3 in SOD1 cells compared to EGFP cells; catalase treatment decreased both indices by an additional 28% following ATP depletion. To test the role of mitochondrial derived superoxide, we also treated a subset of LLC-PK1 cells with the mitochondrial antioxidant, MitoTEMPO. Treatment with MitoTEMPO also decreased ATP depletion induced cytotoxicity in LLC-PK1 cells in a dose dependant manner. These studies indicate that both SOD1 dependent and independent pathways are integral in protection against ATP depletion-recovery mediated cytotoxicity and apoptosis, however more studies are needed to delineate the signaling mechanisms involved.







Similar content being viewed by others
Abbreviations
- EGFP:
-
Enhanced green fluorescent protein
- IM:
-
Injury media
- IR:
-
Ischemia-reperfusion
- LDH:
-
Lactate dehydrogenase
- PBS:
-
Phosphate-buffered saline
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Bonventre JV (1993) Mechanisms of ischemic acute renal failure. Kidney Int 43:1160–1178
Sutton TA, Molitoris BA (1998) Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol 18:490–497
Venkatachalam MA, Jones DB, Rennke HG, Sandstrom D, Patel Y (1981) Mechanism of proximal tubule brush border loss and regeneration following mild renal ischemia. Lab Invest 45:355–365
Molitoris BA (1991) Ischemia-induced loss of epithelial polarity: potential role of the actin cytoskeleton. Am J Physiol 260:F769–F778
Kellerman PS, Bogusky RT (1992) Microfilament disruption occurs very early in ischemic proximal tubule cell injury. Kidney Int 42:896–902
Kellerman PS, Clark RA, Hoilien CA, Linas SL, Molitoris BA (1990) Role of microfilaments in maintenance of proximal tubule structural and functional integrity. Am J Physiol 259:F279–F285
Weight SC, Bell PR, Nicholson ML (1996) Renal ischaemia—reperfusion injury. Br J Surg 83:162–170
Nath KA, Norby SM (2000) Reactive oxygen species and acute renal failure. Am J Med 109:665–678
Lameire N, Vanholder R (2001) Pathophysiologic features and prevention of human and experimental acute tubular necrosis. J Am Soc Nephrol 12(Suppl 17):S20–S32
Sies H (1993) Strategies of antioxidant defense. Eur J Biochem 215:213–219
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Okado-Matsumoto A, Fridovich I (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem 276:38388–38393
Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC (2001) A fraction of yeast Cu, Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem 276:38084–38089
Davies SJ, Reichardt-Pascal SY, Vaughan D, Russell GI (1995) Differential effect of ischaemia-reperfusion injury on anti-oxidant enzyme activity in the rat kidney. Exp Nephrol 3:348–354
Jassem W, Fuggle SV, Rela M, Koo DD, Heaton ND (2002) The role of mitochondria in ischemia/reperfusion injury. Transplantation 73:493–499
Chen Z, Siu B, Ho YS et al (1998) Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol 30:2281–2289
Cruthirds DL, Saba H, MacMillan-Crow LA (2005) Overexpression of manganese superoxide dismutase protects against ATP depletion-mediated cell death of proximal tubule cells. Arch Biochem Biophys 437:96–105
Xiang N, Zhao R, Zhong W (2009) Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells. Cancer Chemother Pharmacol 63:351–362
Yin M, Wheeler MD, Connor HD et al (2001) Cu/Zn-superoxide dismutase gene attenuates ischemia-reperfusion injury in the rat kidney. J Am Soc Nephrol 12:2691–2700
Nilakantan V, Maenpaa C, Jia G, Roman RJ, Park F (2008) 20-HETE-mediated cytotoxicity and apoptosis in ischemic kidney epithelial cells. Am J Physiol Renal Physiol 294:F562–F570
Van Why SK, Mann AS, Ardito T et al (2003) Hsp27 associates with actin and limits injury in energy depleted renal epithelia. J Am Soc Nephrol 14:98–106
Nilakantan V, Liang H, Maenpaa CJ, Johnson CP (2008) Differential patterns of peroxynitrite mediated apoptosis in proximal tubular epithelial cells following ATP depletion recovery. Apoptosis 13:621–633
Maenpaa CJ, Shames BD, Van Why SK, Johnson CP, Nilakantan V (2008) Oxidant-mediated apoptosis in proximal tubular epithelial cells following ATP depletion and recovery. Free Radic Biol Med 44:518–526
Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L (2000) Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet 25:217–222
Park F, Kay MA (2001) Modified HIV-1 based lentiviral vectors have an effect on viral transduction efficiency and gene expression in vitro and in vivo. Mol Ther 4:164–173
Zennou V, Petit C, Guetard D et al (2000) HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173–185
Zufferey R, Donello JE, Trono D, Hope TJ (1999) Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol 73:2886–2892
Dull T, Zufferey R, Kelly M et al (1998) A third-generation lentivirus vector with a conditional packaging system. J Virol 72:8463–8471
Naldini L, Blomer U, Gallay P et al (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263–267
Oh T, Bajwa A, Jia G, Park F (2007) Lentiviral vector design using alternative RNA export elements. Retrovirology 4:38
Park F, Ohashi K, Kay MA (2003) The effect of age on hepatic gene transfer with self-inactivating lentiviral vectors in vivo. Mol Ther 8:314–323
Park F, Ohashi K, Chiu W, Naldini L, Kay MA (2000) Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat Genet 24:49–52
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Zielonka J, Vasquez-Vivar J, Kalyanaraman B (2008) Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3:8–21
Zielonka J, Vasquez-Vivar J, Kalyanaraman B (2006) The confounding effects of light, sonication, and Mn(III)TBAP on quantitation of superoxide using hydroethidine. Free Radic Biol Med 41:1050–1057
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350
Walker NI, Harmon BV, Gobe GC, Kerr JF (1988) Patterns of cell death. Methods Achiev Exp Pathol 13:18–54
Wyllie AH, Kerr JF, Currie AR (1980) Cell death: the significance of apoptosis. Int Rev Cytol 68:251–306
Hiura TS, Li N, Kaplan R et al (2000) The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. J Immunol 165:2703–2711
Comelli M, Di Pancrazio F, Mavelli I (2003) Apoptosis is induced by decline of mitochondrial ATP synthesis in erythroleukemia cells. Free Radic Biol Med 34:1190–1199
Atlante A, Giannattasio S, Bobba A et al (2005) An increase in the ATP levels occurs in cerebellar granule cells en route to apoptosis in which ATP derives from both oxidative phosphorylation and anaerobic glycolysis. Biochim Biophys Acta 1708:50–62
Zhong W, Yan T, Webber MM, Oberley TD (2004) Alteration of cellular phenotype and responses to oxidative stress by manganese superoxide dismutase and a superoxide dismutase mimic in RWPE-2 human prostate adenocarcinoma cells. Antioxid Redox Signal 6:513–522
Keller JN, Kindy MS, Holtsberg FW et al (1998) Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci 18:687–697
Zhou W, Zhang Y, Hosch MS et al (2001) Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology 33:902–914
Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K (1997) Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res 49:681–697
Iwata M, Myerson D, Torok-Storb B, Zager RA (1994) An evaluation of renal tubular DNA laddering in response to oxygen deprivation and oxidant injury. J Am Soc Nephrol 5:1307–1313
Zager RA, Iwata M, Burkhart KM, Schimpf BA (1994) Post-ischemic acute renal failure protects proximal tubules from O2 deprivation injury, possibly by inducing uremia. Kidney Int 45:1760–1768
Doughan AK, Harrison DG, Dikalov SI (2008) Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102:488–496
Schwartzman RA, Cidlowski JA (1993) Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev 14:133–151
Matsuu M, Shichijo K, Okaichi K et al (2003) The protective effect of fermented milk kefir on radiation-induced apoptosis in colonic crypt cells of rats. J Radiat Res (Tokyo) 44:111–115
Metodiewa D, Koska C (2000) Reactive oxygen species and reactive nitrogen species: relevance to cyto(neuro)toxic events and neurologic disorders. An overview. Neurotox Res 1:197–233
Kitazawa M, Wagner JR, Kirby ML, Anantharam V, Kanthasamy AG (2002) Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. J Pharmacol Exp Ther 302:26–35
Doughan AK, Dikalov SI (2007) Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9:1825–1836
Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T et al (2008) Deleterious role of superoxide dismutase in the mitochondrial intermembrane space. J Biol Chem 283:8446–8452
Fullerton HJ, Ditelberg JS, Chen SF et al (1998) Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol 44:357–364
Ying W, Anderson CM, Chen Y et al (2000) Differing effects of copper, zinc superoxide dismutase overexpression on neurotoxicity elicited by nitric oxide, reactive oxygen species, and excitotoxins. J Cereb Blood Flow Metab 20:359–368
Sun JS, Tsuang YH, Huang WC et al (1997) Menadione-induced cytotoxicity to rat osteoblasts. Cell Mol Life Sci 53:967–976
Rezvani HR, Mazurier F, Cario-Andre M et al (2006) Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes. J Biol Chem 281:17999–18007
Acknowledgments
We greatly appreciate the gift of human SOD1 cDNA from Dr. Charles Epstein (University of California, San Francisco). We are also grateful to Dr. Balaraman Kalyanaraman, Jacek Zielonka and the Free Radical Research Center at the Medical College of Wisconsin for help with measurement of 2-OH-E+. This work was supported in part by a pilot and feasibility project in an NIH P50 (1DK-079306-01), an American Heart Association-Scientist Development Grant (0930326G) and departmental funds to V. Nilakantan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, H.L., Arsenault, J., Mortensen, J. et al. Partial attenuation of cytotoxicity and apoptosis by SOD1 in ischemic renal epithelial cells. Apoptosis 14, 1176–1189 (2009). https://doi.org/10.1007/s10495-009-0393-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-009-0393-z