Abstract
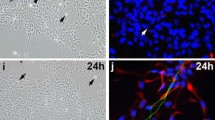
The fungal alkaloid militarinone A (MiliA) was recently found to stimulate neuronal outgrowth in PC-12 cells by persistant activation of pathways that are also involved in NGF-mediated differentiation, namely the PI3-K/PKB and the MEK/ERK pathways. Application of equal concentrations of MiliA to other cells such as the murine neuroblastoma cell line N2a resulted in immediate onset of apoptosis by nuclear translocation of apoptosis inducing factor (AIF), activation of caspases and c-Jun/AP-1 transcription factor without an intermediate differentiated phenotype, although minor transient phosphorylation of PKB and MAPK as well as activation of NF-κB were also observed. Translocation of AIF was preceded by p53 phosphorylation at Ser15 and blocked by pifithrin α, a known inhibitor of p53-transcriptional activity. We here show that both cell types activate the same pathways albeit in different time scales. This is mainly due to contrasting basal expression levels of p53, which in turn regulates expression of AIF. In PC-12 cells, continuous activation of these pathways after prolonged treatment with 40 μM MiliA first led to up-regulation of p53, phosphorylation of p53, release of AIF from mitochondria and its translocation into the nucleus. Additionally, also activation of the c-Jun/AP-1 transcription factor was observed, and PC-12 cells subsequently underwent apoptosis 48–72 h post-treatment. We report that similar pathways working on different levels are able to initially shape very divergent cellular responses.









Similar content being viewed by others
References
Blesch A (2006) Neurotrophic factors in neurodegeneration. Brain Pathol 16:295–303
Pollack SJ, Harper SJ (2002) Small molecule Trk receptor agonists and other neurotrophic factor mimetics. Curr Drug Targets CNS Neurol Disord 1:59–80
Riese U, Ziegler E, Hamburger M (2004) Militarinone A induces differentiation in PC12 cells via MAP and Akt kinase signal transduction pathways. FEBS Lett 577:455–459
Frebel K, Wiese S (2006) Signalling molecules essential for neuronal survival and differentiation. Biochem Soc Trans 34:1287–1290
Vaudry D, Stork PJS, Lazarovici P, Eiden LE (2002) Differentiation pathway in PC12 Cells. Sci. STKE. http://stke.sciencemag.org/cgi/cm/stkecm;CMP_8038
Philpott KL, McCarthy MJ, Klippel A, Rubin LL (1997) Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol 139:809–815
Kimura K, Hattori S, Kabuyama Y et al (1994) Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem 269:18961–18967
Rubinfeld H, Seger R (2005) The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol 31:151–174
Kimmelman AC, Nunez Rodriguez N, Chan AM (2002) R-Ras3/M-Ras induces neuronal differentiation of PC12 cells through cell-type-specific activation of the mitogen-activated protein kinase cascade. Mol Cell Biol 22:5946–5961
Modjtahedi N, Giordanetto F, Madeo F, Kroemer G (2006) Apoptosis-inducing factor: vital and lethal. Trends Cell Biol 16:264–272
Porter AG, Urbano AG (2006) Does apoptosis-inducing factor (AIF) have both life and death functions in cells? Bioessays 28:834–843
Cregan SP, Dawson VL, Slack RS (2004) Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 23:2785–2796
Cande C, Cohen I, Daugas E et al (2002) Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie 84:215–222
Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2:589–598
Urbano A, Lakshmanan U, Choo PH et al (2005) AIF suppresses chemical stress-induced apoptosis and maintains the transformed state of tumor cells. Embo J 24:2815–2826
Stambolsky P, Weisz L, Shats I et al (2006) Regulation of AIF expression by p53. Cell Death Differ 13:2140–2149
Mayo LD, Donner DB (2002) The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci 27:462–467
Wymann MP, Zvelebil M, Laffargue M (2003) Phosphoinositide 3-kinase signalling—which way to target? Trends Pharmacol Sci 24:366–376
Vousden KH, Lu X (2002) Live or let die: the cell’s response to p53. Nat Rev Cancer 2:594–604
Hofseth LJ, Hussain SP, Harris CC (2004) p53: 25 years after its discovery. Trends Pharmacol Sci 25:177–181
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B (1997) A model for p53-induced apoptosis. Nature 389:300–305
D’Autreaux B, Toledano MB (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8:813–824
Gloire G, Legrand-Poels S, Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72:1493–1505
Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB (2006) Active defense under oxidative stress. The antioxidant responsive element. Biochemistry (Mosc) 71:962–974
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
Hess J, Angel P, Schorpp-Kistner M (2004) AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117:5965–5973
Rossler OG, Steinmuller L, Giehl KM, Thiel G (2002) Role of c-Jun concentration in neuronal cell death. J Neurosci Res 70:655–664
Karin M, Lin A (2002) NF-kappaB at the crossroads of life and death. Nat Immunol 3:221–227
van Delft MF, Huang DC (2006) How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res 16:203–213
Kuenzi P, Schneider P, Dobbelaere DA (2003) Theileria parva-transformed T cells show enhanced resistance to Fas/Fas ligand-induced apoptosis. J Immunol 171:1224–1231
Heussler VT, Kuenzi P, Fraga F, Schwab RA, Hemmings BA, Dobbelaere DA (2001) The Akt/PKB pathway is constitutively activated in Theileria-transformed leucocytes, but does not directly control constitutive NF-kappaB activation. Cell Microbiol 3:537–550
Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260
Botteron C, Dobbelaere D (1998) AP-1 and ATF-2 are constitutively activated via the JNK pathway in Theileria parva-transformed T-cells. Biochem Biophys Res Commun 246:418–421
Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73:2424–2428
Gogvadze V, Orrenius S, Zhivotovsky B (2006) Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta 1757:639–647
Schmidt K, Gunther W, Stoyanova S, Schubert B, Li Z, Hamburger M (2002) Militarinone A, a neurotrophic pyridone alkaloid from Paecilomyces militaris. Org Lett 4:197–199
Ly JD, Grubb DR, Lawen A (2003) The mitochondrial membrane potential (deltapsi(m)) in apoptosis: an update. Apoptosis 8:115–128
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Bar J, Lukaschuk N, Zalcenstein A, Wilder S, Seger R, Oren M (2005) The PI3K inhibitor LY294002 prevents p53 induction by DNA damage and attenuates chemotherapy-induced apoptosis. Cell Death Differ 12:1578–1587
Bouleau S, Parvu-Ferecatu I, Rodriguez-Enfedaque A et al (2007) Fibroblast growth factor 1 inhibits p53-dependent apoptosis in PC12 cells. Apoptosis 12:1377–1387
Nakanishi M, Ozaki T, Yamamoto H et al (2007) NFBD1/MDC1 associates with p53 and regulates its function at the crossroad between cell survival and death in response to DNA damage. J Biol Chem 282:22993–23004
Komarov PG, Komarova EA, Kondratov RV et al (1999) A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285:1733–1737
Franzoso G, Zazzeroni F, Papa S (2003) JNK: a killer on a transcriptional leash. Cell Death Differ 10:13–15
Lopez-Maderuelo MD, Fernandez-Renart M, Moratilla C, Renart J (2001) Opposite effects of the Hsp90 inhibitor Geldanamycin: induction of apoptosis in PC12, and differentiation in N2A cells. FEBS Lett 490:23–27
Cheng Y, Schneider B, Riese U, Schubert B, Li Z, Hamburger M (2006) (+)-N-Deoxymilitarinone A, a neuritogenic pyridone alkaloid from the insect pathogenic fungus Paecilomyces farinosus. J Nat Prod 69:436–438
Symons M, Takai Y (2001) Ras GTPases: singing in tune. Sci STKE 2001: PE1
Wennerberg K, Rossman KL, Der CJ (2005) The Ras superfamily at a glance. J Cell Sci 118:843–846
Xu J, Ji LD, Xu LH (2006) Lead-induced apoptosis in PC 12 cells: involvement of p53, Bcl-2 family and caspase-3. Toxicol Lett 166:160–167
Sabbatini P, McCormick F (1999) Phosphoinositide 3-OH kinase (PI3K) and PKB/Akt delay the onset of p53-mediated, transcriptionally dependent apoptosis. J Biol Chem 274:24263–24269
Song G, Ouyang G, Bao S (2005) The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9:59–71
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Dobbelaere DA, Kuenzi P (2004) The strategies of the Theileria parasite: a new twist in host-pathogen interactions. Curr Opin Immunol 16:524–530
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Küenzi, P., Kiefer, S., Koryakina, A. et al. Promotion of cell death or neurite outgrowth in PC-12 and N2a cells by the fungal alkaloid militarinone A depends on basal expression of p53. Apoptosis 13, 364–376 (2008). https://doi.org/10.1007/s10495-008-0185-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0185-x