Abstract
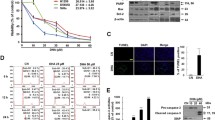
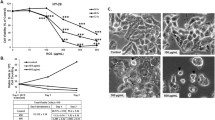
We reported previously that 7β-hydroxysitosterol and 7β-hydroxycholesterol induced apoptosis in Caco-2 cells. Apoptosis caused by 7β-hydroxysitosterol but not by 7β-hydroxycholesterol was related to a caspase-dependent process. In the present report, we compared the effects of both compounds on mitochondria integrity and on various modulators of apoptosis. When Caco-2 cells were exposed to both hydroxysterols, no changes in Bcl-2 and Bax expressions were detected indicating a Bcl-2/Bax-independent cell death pathway, whereas loss of mitochondrial membrane potential and cytochrome c release were observed. Endonuclease G expression and enhanced production of reactive oxygen species were detected in 7β-hydroxycholesterol treated cells, but not with 7β-hydroxysitosterol. Loss of mitochondrial membrane potential and cell death produced by both hydroxysterols were prevented by vitamin C. Lysosomal membrane integrity was altered with both hydroxysterols, but 7β-hydroxysitosterol was significantly more active on than 7β-hydroxycholesterol. Both hydroxysterols induced apoptosis by mitochondrial membrane permeabilization. However, 7β-hydroxycholesterol exhibited a specific enhancement of oxidative stress and of endonuclease G expression despite its closely related chemical structure with 7β-hydroxysitosterol. The two hydroxysterols exhibit different lipophilic properties which may explain their different biological effects.
Similar content being viewed by others
References
Lea LJ, Hepburn PA, Wolfreys AM, Baldrick P (2004) Safety evaluation of phytosterol esters. Part 8. Lack of genotoxicity and subchronic toxicity with phytosterol oxides. Food Chem Toxicol 42:771–83
Brown AJ, Jessup W (1999) Oxysterols and atherosclerosis. Atherosclerosis 142:1–8
Schroepfer GJ Jr. (2000) Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev 80:361–54
Maguire L, Konoplyannikov M, Ford A, Maguire AR, O’Brien NM (2003) Comparison of the cytotoxic effects of beta-sitosterol oxides and a cholesterol oxide, 7beta-hydroxycholesterol, in cultured mammalian cells. Br J Nutr 90:767–75.
Ryan E, Chopra J, McCarthy F, Maguire AR, O’Brien NM (2005) Qualitative and quantitative comparison of the cytotoxic and apoptotic potential of phytosterol oxidation products with their corresponding cholesterol oxidation products. Br J Nutr 94:443–51
Tapiero H, Townsend DM, Tew KD (2003) Phytosterols in the prevention of human pathologies. Biomed Pharmacother 57:321–25
de Jong A, Plat J, Mensink RP (2003) Metabolic effects of plant sterols and stanols. J Nutr Biochem 14:362–69
Kroemer G, Martin SJ (2005) Caspase-independent cell death. Nat Med 11:725–30
Kroemer G, Jaattela M (2005) Lysosomes and autophagy in cell death control. Nat Rev Cancer 5:886–97
Armstrong JS (2006) Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioessays 28:253–60
Antonsson B (2004) Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol Cell Biochem 256:141–55
Henry-Mowatt J, Dive C, Martinou JC, James D (2004) Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene 23:2850–860
Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signalling. Biochimie 84:131–41
Roussi S, Winter A, Gosse F et al (2005) Different apoptotic mechanisms are involved in the antiproliferative effects of 7beta-hydroxysitosterol and 7beta-hydroxycholesterol in human colon cancer cells. Cell Death Differ 12:128–35
Zhang X, Julien-David D, Miesch M et al (2005) Identification and quantitative analysis of beta-sitosterol oxides in vegetable oils by capillary gas chromatography-mass spectrometry. Steroids 70:896–06
Boudard D, Vasselon C, Bertheas MF et al (2002) Expression and prognostic significance of Bcl-2 family proteins in myelodysplastic syndromes. Am J Hematol 70:115–25
Egler RA, Fernandes E, Rothermund K et al (2005) Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene 24:8038–050
Yuan XM, Li W, Dalen H et al (2002) Lysosomal destabilization in p53-induced apoptosis. Proc Nat Acad Sci USA 99:6286–291
Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC (1993) Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res 53:4701–714
Kim R, Emi M, Tanabe K, Murakami S, Uchida Y, Arihiro K (2004) Regulation and interplay of apoptotic and non-apoptotic cell death. J Pathol 208:319–26
Miura Y, Takahara K, Murata Y, Utsumi K, Tada M, Takahata K (2004) Docosahexaenoic acid induces apoptosis via the Bax-independent pathway in HL-60 cells. Biosci Biotechnol Biochem 68:2415–417
Shimizu S, Kanaseki T, Mizushima N et al (2004) Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 6:1221–228
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13:1423–433
van Loo GS (2001) Endonuclease G A mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ 8:1136–142.
Chen Q, Chai YC, Mazumder S et al (2003) The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ 10:323–34
Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL (2004) Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens 22:1141–149
Wenzel U, Nickel A, Kuntz S, Daniel H (2004) Ascorbic acid suppresses drug-induced apoptosis in human colon cancer cells by scavenging mitochondrial superoxide anions. Carcinogenesis 25:703–12
Antunes F, Cadenas E, Brunk UT (2001) Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem J 356:549–55
Hatch GM, Oskin A, Vance DE (1993) Involvement of the lysosome in the catabolism of intracellular lysophosphatidylcholine and evidence for distinct pools of lysophosphatidylcholine. J Lipid Res 34:1873–881
Breckenridge DG, Xue D (2004) Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol 16:647–52
Prunet C, Lemaire-Ewing S, Menetrier F, Neel D, Lizard G (2005) Activation of caspase-3-dependent and -independent pathways during 7-ketocholesterol- and 7beta-hydroxycholesterol-induced cell death: a morphological and biochemical study. J Biochem Mol Toxicol 19:311–26
Enns GM (2003) The contribution of mitochondria to common disorders. Mol Genet Metab 80:11–6
Lizard G, Lemaire S, Monier S, Gueldry S, Neel D, Gambert P (1997) Induction of apoptosis and of interleukin-1beta secretion by 7beta-hydroxycholesterol and 7-ketocholesterol: partial inhibition by Bcl-2 overexpression. FEBS Lett 419:276–80
Benimetskaya L, Lai JC, Khvorova A et al (2004) Relative Bcl-2 independence of drug-induced cytotoxicity and resistance in 518A2 melanoma cells. Clin Cancer Res 10:8371–379
Yu J, Zhang L (2005) The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 331:851–58
Karczewski JM, Vet JA, Hessels D, Noordhoek J (2000) p53-independent apoptosis induced by menadione in the human colon carcinoma cell line Caco-2. Ann N Y Acad Sci 15:275–78
Lemaire-Ewing S, Prunet C, Montange T et al (2005) Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol Toxicol 21:97–14
Ryan L, O’Callaghan YC, O’Brien NM (2005) The role of the mitochondria in apoptosis induced by 7beta-hydroxycholesterol and cholesterol-5beta, 6beta-epoxide. Br J Nutr 94:519–25
Tsujimoto Y (2003) Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol 195:158–67
Kim R, Emi M, Tanabe K (2006) Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol 57:545–53
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:37–6
Chwieralski CE, Welte T, Buhling F (2006) Cathepsin-regulated apoptosis. Apoptosis 11:143–49
Yuan XM, Li W, Brunk UT, Dalen H, Chang YH, Sevanian A (2000) Lysosomal destabilization during macrophage damage induced by cholesterol oxidation products. Free Radic Biol Med 28:208–18
Miguet-Alfonsi C, Prunet C, Monier S et al (2002) Analysis of oxidative processes and of myelin figures formation before and after the loss of mitochondrial transmembrane potential during 7beta-hydroxycholesterol and 7-ketocholesterol-induced apoptosis: comparison with various pro-apoptotic chemicals. Biochem Pharmacol 64: 527–41
Massey JB (2006) Membrane and protein interactions of oxysterols. Curr Opin Lipidol 17:296–01
Awad AB, Chen YC, Fink CS, Hennessey T (1996) beta-Sitosterol inhibits HT-29 human colon cancer cell growth and alters membrane lipids. Anticancer Res 16:2797–804
Bloch KE (1983) Sterol structure and membrane function. CRC Crit Rev Biochem 14:47–2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roussi, S., Gossé, F., Aoudé-Werner, D. et al. Mitochondrial perturbation, oxidative stress and lysosomal destabilization are involved in 7β-hydroxysitosterol and 7β-hydroxycholesterol triggered apoptosis in human colon cancer cells. Apoptosis 12, 87–96 (2007). https://doi.org/10.1007/s10495-006-0485-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-0485-y