Abstract
Borrelia miyamotoi, a spirochete found in the hard tick Ixodes ricinus, is thought to cause relapsing fever. The disease caused by this bacterium can manifest with high fever, fatigue and other symptoms. It may also lead to central nervous system involvement with symptoms similar to meningoencephalitis. DNA from ticks from the greater Augsburg region in Germany was subjected to qPCR for Borrelia spp., followed by nested PCR and subsequent sequencing for species identification of the qPCR positive samples. From 112 ticks, 20 were found to be positive for Borrelia. The DNA sequenced showed 50% Borrelia afzelli, 15% Borrelia garinii, 5% Borrelia valaisiana and one sequence was identified as Borrelia miyamotoi. The positive identification of Borrelia miyamotoi is unlikely to be due to contamination. In conclusion, Borrelia miyamotoi has been found in a tick in the Augsburg region for the first time. This follows on from previous reports of a low incidence of this bacterium in southern Germany around Lake Constance and in the Munich region. This infectious agent should be taken into account when patients present with recurring fever or neurological symptoms which cannot be otherwise explained. Tick-borne relapsing fever should now be considered as a cause of such symptoms and medical professionals should contemplate differential Borrelia testing when presented with corresponding symptoms.
Similar content being viewed by others
Introduction
The different Borrelia species can be separated into two distinct groups; those known to cause Lyme borreliosis (LB) and those which can induce relapsing fever (RF). Most members of the RF group of Borrelia are transmitted by soft ticks whereas hard ticks of the genus Ixodes are the vector for LB bacteria (Krause et al. 2015). Contrary to this, a putative causative agent of relapsing fever, Borrelia miyamotoi, has been found in Ixodes ticks. It is a recently identified species thought to cause relapsing fever in humans after infection, although this is not well-documented (Platonov et al. 2011; Krause et al. 2015; Telford III et al. 2015). This species of Borrelia was first characterized in Japan in 1995 in Ixodes persulcatus ticks and in mouse blood (Fukunaga et al. 1995). DNA from this novel RF spirochete has subsequently been detected in ixodid ticks from the USA and in Europe, with reports of B. miyamotoi infection of ticks in the central Rhine valley in Germany, as well as in the city of Constance further south along the Rhine close to its source (Scoles et al. 2001; Richter et al. 2003; Crowder et al. 2014) in Berlin-Brandenburg (Schreiber et al. 2014) and in Munich (Venczel et al. 2016). It has also been identified in Estonia (Geller et al. 2012), Poland (Sytykiewicz et al. 2015), Sweden (Fraenkel et al. 2002; Wilhelmsson et al. 2010), the Netherlands (Jahfari et al. 2016) and many other European countries (Siński et al. 2016). Relapsing fever in humans caused by B. miyamotoi has been described in Russia (Platonov et al. 2011) and other countries. The disease caused by infection with this organism tends to manifest with high fever (exceeding 40 °C), fatigue, headache, chills, myalgia, arthralgia and nausea [data from (Krause et al. 2015)]. The fever associated with the infection lasts approximately one week, regardless of antibiotic treatment, but other symptoms such as fatigue may persist for a longer period. Untreated infections will show 2–3 episodes of fever with a mean interval of 9 days. There have been a few other reported cases in the USA (Gugliotta et al. 2013), Germany (Boden et al. 2016) and the Netherlands (Lee et al. 2014; Hovius et al. 2017) of B. miyamotoi related infection with central nervous system involvement but without fever, presenting as meningoencephalitis. The patients initially showed of symptoms of this disease, followed by a progressive decline in cognition and an unsteady gait. In these cases, antibiotic therapy restored complete neural function 1 month after the start of treatment. Relapsing fever cases have also been retrospectively reported in Japan (Sato et al. 2014).
In order to establish an overview of bacterial infestation of ticks in the Augsburg region, specimens were collected and examined for the prevalence and identity of all Borrelia spp. in this area. Ticks are active and numerous in this region, with many opportunities for tick bite of humans offered by the diverse outdoor leisure activities available. There is a substantial wildlife population, providing host vectors for larvae through to the adult stage of the tick. Tick-borne disease is an increasing health concern in Europe and a survey of the incidence of Borrelia in particular could aid physicians in diagnosis of Lyme borreliosis or other tick-borne disease such as Borrelia-related relapsing fevers.
Materials and methods
Tick collection
The laboratory put out a call to the general public in the spring of 2016 to send in ticks taken from any source, including household pets and wild animals. Over the course of the spring and summer months, 350 ticks were acquired. These were sorted geographically into groups for the greater Augsburg region and a selection made to maximize the local distribution of ticks to be investigated. Before analysis the ticks were stored in 70% ethanol at − 20 °C. Of these 350 ticks, 120 were selected for further analysis. The ticks were not examined morphologically, but were subjected to PCR analysis and sequencing for species identification (see below).
DNA extraction
DNA was extracted from the 120 selected ticks using either the BlackPrep Tick DNA Kit (Analytik Jena, Jena, Germany) for large or engorged ticks or the Qiagen Viral RNA Mini Kit (Qiagen, Hilden, Germany) for smaller examples. All kits were used according to the manufacturers’ instructions. Prior to extraction the ticks were washed briefly in fresh 70% ethanol. The extracted DNA was stored at − 20 °C prior to polymerase chain reaction (PCR) analysis.
PCR and qPCR analysis
The DNA was initially assessed for the presence of the tick mitochondrial 16S rRNA gene in order to confirm that the organism processed was indeed a tick. The primers used were: Tick16Sfor 5′-TGACTATACGAAGGTATTGAAATAAG-3′ and tick16Srev 5′-TCCTAATCCAACATCGAGGTC-3′. These primers produce a 350 bp product. The DNA extract (3 µL) was subjected to PCR in 1× green GoTaqG2 PCR buffer (Promega, Mannheim, Germany) with 2.5 mM MgCl2, 0.1 mM dNTPs, 1 µM each primer and 1 unit of Taq (GoTaq, Promega) per reaction in 25 µL. Initial denaturation was carried out at 95 °C for 5 min, followed by 15 cycles of denaturation at 95 °C for 30 s, annealing and extension 60 °C for 2 min. This was immediately followed by 30 cycles of denaturation at 95 °C for 10 s, annealing at 56 °C for 10 s, extension/elongation at 72 °C for 30 s. The final extension was performed at 72 °C for 8 min with subsequent cooling at 8 °C. The reaction was analysed on a 2% agarose gel stained with RedSafe (Intron Biotechnology, distributed by HiSS Diagnostics, Freiburg, Germany) for the presence of the appropriately-sized 350 bp product. Only samples containing this band were further analysed. Positive and negative controls plus a PCR control were run for each analysis to rule our contamination or failure of the PCR. Selected PCR products were sequenced externally in order to identify the tick species (Eurofins, Ebersberg, Germany). The results obtained were submitted to the BLAST program at the NCBI to identify sequence similarities.
The tick DNA samples were screened for the presence of Borrelia DNA in the GeneProof Borrelia burgdorferi PCR Kit (GeneProof, Brno, Czech Republic). This qPCR kit is able to identify many Borrelia species but does not differentiate between them. The kit was used according to the manufacturers’ instructions. The reactions were analysed in the qTower 2.2 (Analytik Jena) with the following protocol: 37 °C for 2 min, 95 °C for 10 min, followed by 45 cycles of 95 °C for 5 s, 60 °C for 40 s and 72 °C for 20 s. The data were collected in the FAM and HEX channels for Borrelia DNA and the internal control respectively. The results were examined for the presence of Borrelia DNA and the internal control to confirm successful amplification. A Ct value of < 38 was counted as positive in the subsequent analysis. Since the qPCR kit does not distinguish between Borrelia species further PCR reactions were performed.
Species identification and sequencing
The Borrelia positive DNA samples identified by the GeneProof Borrelia kit were subjected to PCR using primers which recognize a region of the 16S–23S intergenic spacer (IGS) in the Borrelia genome. This is a highly variable region and enables a single primer pair to amplify products of varying size depending on the species of Borrelia present in the tick. A nested PCR was carried out to enhance sensitivity and aid identification. Each 25 µL reaction was carried out in 1× GoTaqG2 PCR buffer (Promega; colourless buffer in the first PCR, green buffer in the second), with 2.5 mM MgCl2, 0.2 mM dNTPs, 0.5 µM each primer, 1U Taq (Promega). Five µL of the tick DNA extract was used. The outer primer pair was: IGS_F1 5′-GTATGTTTAGTGAGGGGGGTG-3′ and IGS_R1 5′-GGATCATAGCTCAGGTGGTTAG-3′, the inner primers IGS_F2 5′-AGGGGGGTGAAGTCGTAACAAG-3′ and IGS_R2 5′-GTCTGATAAACCTGAGGTCGGA-3′ (Kjelland et al. 2015; Quarsten et al. 2015). The outer PCR was run with an initial denaturation step at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 61 °C for 30 s, extension/elongation at 72 °C for 1 min and a final extension at 72 °C for 8 min. 5 µL of the product resulting from the outer PCR was used as a template in the inner PCR with an amplification reaction as follows: Denaturation at 95 °C, 2 min; 30 cycles of denaturation 95 °C for 30 s, annealing 65 °C for 30 s, extension/elongation at 72 °C for 1 min and with a final extension at 72 °C for 8 min. The resulting products were analysed on an agarose gel. The bands were cut out of the gel and cleaned using the E.Z.N.A. Gel Extraction Kit (VWR Omega, Darmstadt, Germany) and sent to Seqlab (Göttingen, Germany) for sequencing. The results obtained were submitted to the BLAST program at the NCBI to identify sequence similarities.
Results
Out of the 120 selected ticks, 112 samples examined showed a positive result for the tick 16S rRNA gene (data not shown) and were used in further experiments. Ten of these samples were sequenced to identify the tick species. All tested PCR products matched the NCBI DNA sequences for Ixodes ricinus, as can be expected since this tick species is the most common found in this region of Germany (data not shown). As can be seen in Table 1, of the 112 ticks examined by qPCR, 20 (17.9%) were found to be positive for Borrelia spp. DNA (second column, Borrelia qPCR). This percentage falls within the range indicated by the Robert-Koch Institute for Germany, with a country-wide infection rate of 5-35% dependent on region (data from 2014).
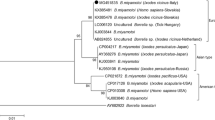
These samples were then subjected to nested IGS PCR (Fig. 1) and any resulting bands were excised from the gel, the DNA extracted and commercially sequenced. Three of the qPCR positive samples showed a negative IGS PCR result (ZK#18, 73 and 303). These may have been false positive results or DNA from a Borrelia species recognized by the qPCR kit which is not amplified in the nested PCR. These qPCR primers were designed for maximum recognition of Borrelia species and the kit is certified by the manufacturer to recognize Borrelia sensu strictu, B. garinii, B. afzelli, B. andersonii, B. bisetii, B. valaisianae, B. lusitaniae, B. japonica, B. tanukii, B. turdii, B. sinica, B. miyamotoi and B. mayonii. The IGS primers recognize these species; however mismatches cannot be excluded with 100% certainty. It is also possible that species sensitivity in the IGS PCR may vary to that of the commercial qPCR kit, thus masking a qPCR positive result.
IGS-nested PCR and identification of Borrelia species. DNA extracted from ticks positive for Borrelia spp. DNA in qPCR was subjected to a nested PCR using primers designed to bind to the intergenic spacer region of the genome. The resulting products were analysed by gel electrophoresis followed by excision of the bands, DNA purification and commercial sequencing. The sequencing results are indicated as follows, g: B. garinii; g1: B. garinii and B. valaisianae; a: B. afzelii; m: B. miyamotoi; u: unidentified; *negative result. +: indicates the positive control (Borrelia burgdorferi DNA), is the negative control (Bartonella DNA) and ntc indicates the no-template-controls used in both steps of the nested PCR (water instead of DNA)
The purified DNA from the IGS positive samples was successfully sequenced in all but two cases, where the DNA was either not of sufficient quantity or quality to allow the reaction to proceed. Of these, 50% (10/20) were found to be B. afzelii, 15% (3/20) were B. garinii, 5% (1/20) was B. valaisianae and one (5%) was identified as Borrelia miyamotoi. This sample was re-examined in a second independent IGS PCR reaction followed by sequencing and the same result was achieved, with a 99% sequence match over 328 nucleotides (one gap and one mismatch compared to the ribosomal RNA 16S-23S intergenic spacer region of B. miyamotoi, isolate Z58 Accession Number KP202177.1 (Fig. 2). To our knowledge, this is the first report of B. miyamotoi in a tick in the greater Augsburg region. We can exclude laboratory contamination since at the time of DNA extraction there was neither B. miyamotoi DNA nor bacteria in culture in the laboratory. Moreover, at the time of tick DNA extraction, this DNA had never been present in the laboratory. The positive control showed a band of the expected size for B. burgdorferi whereas the negative controls, both extraction and PCR based no-template-control showed no amplification products. We are confident that this is the first identification of this species in the area.
The distribution of ticks negative and positive for Borrelia DNA was mapped using BatchGeo software and can be seen in Fig. 3. There was no obvious pattern to the location of the positive ticks, at least within this small sample.
Location of Borrelia DNA positive and negative ticks in the Augsburg region. The results from the PCR analysis were mapped using BatchGeo software. The left map illustrates an overview of the ticks tested. The right map shows the greater Augsburg region in detail. *The location of the Borrelia miyamotoi positive tick. Pale blue pins indicate negative results, dark orange pins the positive ticks. There is some overlap of markers where ticks from similar locations were investigated. The map can be viewed at: https://de.batchgeo.com/map/1413c3497406be0fc00523b430de14b6. (Color figure online)
Discussion
Borrelia miyamotoi infection of a human vector can lead to relapsing fever or meningoencephalitis. This bacterial infection is not well-known among physicians or well-described in Europe and diagnosis may prove difficult. This is the first report of this species of the Borrelia genus in a tick in the Augsburg region in southern Germany. The infectious agent was found in a tick taken from a hedgehog and as such is unlikely to have been transported directly from a different geographical location. A hedgehog may travel up to 3–4 km a night searching for food but it has a defined territory and will not stray further (Morris 1988). Hedgehogs are known to contribute to tick-borne disease spread and maintenance (Jahfari et al. 2017). The B. miyamotoi will presumably have been imported into the area in a tick vector on another species. B. miyamotoi, unlike B. burgdorferi, can be passed between the different stages of tick in the lifecycle without an intermediate mammalian host (van Duijvendijk et al. 2016), but will also infect said host during a blood meal. The bacterium may have already been present in the region but until now remained undetected in ticks. The identification of this species in this area could be important for medical professionals when considering diagnosis of potential tick-borne diseases. Symptoms of relapsing fever or indeed neurological symptoms of meningoencephalitis may not initially be attributed to Borrelia infection if the infectious agent is rare. It must however now come into consideration when patients present with these symptoms and a suitable diagnostic process should confirm the presence or absence of the spirochete. Diagnosis may be performed with conventional antibody analysis or through PCR as above. However, the diagnostic procedure might prove difficult since antibody detection occurs only later during the disease, and the available antibody test assays for serum and cerebrospinal fluid (CSF) for antibody index assessment might not cover B. miyamotoi. Furthermore, molecular detection in the CSF might lack sensitivity (Aguero-Rosenfeld et al. 2005). Further analysis of ticks from this region will lead to an estimate of the prevalence of this novel Borrelia species and others in southern Germany.
Conclusion
This is the first report of B. miyamotoi in an I. ricinus tick in the Augsburg region of southern Germany. The identification of this bacterium may suggest a low level prevalence of the pathogen in this area. This has implications for medical practitioners with respect to diagnosis of diseases potentially caused by this spirochete which up until this time point would not have been under consideration.
Abbreviations
- LB:
-
Lyme borreliosis
- RF:
-
Relapsing fever
- PCR:
-
Polymerase chain reaction
- qPCR:
-
Quantitative PCR
- IGS:
-
Intergenic spacer
References
Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP (2005) Diagnosis of lyme borreliosis. Clin Microbiol Rev 18:484–509. https://doi.org/10.1128/CMR.18.3.484-509.2005
Boden K, Lobenstein S, Hermann B et al (2016) Borrelia miyamotoi—associated Neuroborreliosis in Immunocompromised Person. Emerg Infect Dis 22:1617–1620. https://doi.org/10.3201/eid2209.152034
Crowder CD, Carolan HE, Rounds MA et al (2014) Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg Infect Dis 20:1678–1682. https://doi.org/10.3201/eid2010.131583
Fraenkel C, Garpmo U, Berglund J (2002) Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J Clin Microbiol 40:3308–3312. https://doi.org/10.1128/JCM.40.9.3308
Fukunaga M, Takahashi Y, Tsuruta Y et al (1995) Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol 45:804–810. https://doi.org/10.1099/00207713-45-4-804
Geller J, Nazarova L, Katargina O et al (2012) Detection and genetic characterization of relapsing fever spirochete Borrelia miyamotoi in Estonian ticks. PLoS ONE. https://doi.org/10.1371/journal.pone.0051914
Gugliotta JL, Goethert HK, Berardi VP, Telford SR (2013) Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 368:240–245. https://doi.org/10.1056/NEJMoa1209039
Hovius JWR, de Wever B, Sohne M et al (2017) A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 382:658. https://doi.org/10.1016/S0140-6736(13)61644-X
Jahfari S, Hofhuis A, Fonville M et al (2016) Molecular detection of tick-borne pathogens in humans with tick bites and erythema migrans, in the Netherlands. PLoS Negl Trop Dis 10:1–15. https://doi.org/10.1371/journal.pntd.0005042
Jahfari S, Ruyts SC, Frazer-Mendelewska E et al (2017) Melting pot of tick-borne zoonoses: the European hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles urban and suburban areas. Parasit Vectors 10:134. https://doi.org/10.1186/s13071-017-2065-0
Kjelland V, Rollum R, Korslund L et al (2015) Borrelia miyamotoi is widespread in Ixodes ricinus ticks in southern Norway. Ticks Tick Borne Dis 6:516–521. https://doi.org/10.1016/j.ttbdis.2015.04.004
Krause PJ, Fish D, Narasimhan S, Barbour AG (2015) Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect 21:631–639. https://doi.org/10.1016/j.cmi.2015.02.006
Lee SH, Vigliotti JS, Vigliotti VS et al (2014) DNA sequencing diagnosis of off-season spirochetemia with low bacterial density in Borrelia burgdorferi and Borrelia miyamotoi infections. Int J Mol Sci 15:11364–11386. https://doi.org/10.3390/ijms150711364
Morris PA (1988) A study of home range and movements in the hedgehog (Erinaceus europaeus). J Zool 214:433–449. https://doi.org/10.1111/j.1469-7998.1988.tb03751.x
Platonov AE, Karan LS, Kolyasnikova NM et al (2011) Humans Infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis 17:1816–1823. https://doi.org/10.3201/eid1710.101474
Quarsten H, Skarpaas T, Fajs L et al (2015) Tick-borne bacteria in Ixodes ricinus collected in southern Norway evaluated by a commercial kit and established real-time PCR protocols. Ticks Tick Borne Dis 6:538–544. https://doi.org/10.1016/j.ttbdis.2015.04.008
Richter D, Schlee DB, Matuschka FR (2003) Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg Infect Dis 9:697–701. https://doi.org/10.3201/eid0906.020459
Sato K, Takano A, Konnai S et al (2014) Human infections with Borrelia miyamotoi, Japan. Emerg Infect Dis. https://doi.org/10.3201/eid2008.131761
Schreiber C, Krücken J, Beck S et al (2014) Pathogens in ticks collected from dogs in Berlin/Brandenburg, Germany. Parasit Vectors 7:535. https://doi.org/10.1186/s13071-014-0535-1
Scoles GA, Papero M, Beati L, Fish D (2001) A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector-Borne Zoonotic Dis 1:21–34
Siński E, Welc-Falęciak R, Zajkowska J (2016) Borrelia miyamotoi: a human tick-borne relapsing fever spirochete in Europe and its potential impact on public health. Adv Med Sci 61:255–260. https://doi.org/10.1016/j.advms.2016.03.001
Sytykiewicz H, Karbowiak G, Chorostowska-Wynimko J et al (2015) Coexistence of Borrelia burgdorferi s.l. genospecies within Ixodes ricinus ticks from central and eastern Poland. Acta Parasitol 60:654–661. https://doi.org/10.1515/ap-2015-0093
Telford SR III, Goethert HK, Molloy PJ et al (2015) Borrelia miyamotoi disease: neither lyme disease nor relapsing fever. Clin Lab Med 35:867–882. https://doi.org/10.1016/j.cll.2015.08.002
van Duijvendijk G, Coipan C, Wagemakers A et al (2016) Larvae of Ixodes ricinus transmit Borrelia afzelii and B. miyamotoi to vertebrate hosts. Parasit Vectors 9:97. https://doi.org/10.1186/s13071-016-1389-5
Venczel R, Knoke L, Pavlovic M et al (2016) A novel duplex real-time PCR permits simultaneous detection and differentiation of Borrelia miyamotoi and Borrelia burgdorferi sensu lato. Infection 44:47–55. https://doi.org/10.1007/s15010-015-0820-8
Wilhelmsson P, Fryland L, Börjesson S et al (2010) Prevalence and diversity of Borrelia species in ticks that have bitten humans in Sweden. J Clin Microbiol 48:4169–4176. https://doi.org/10.1128/JCM.01061-10
Acknowledgements
The authors would like to thank Vanessa Senteck and Joanna Schmucker for excellent technical assistance with DNA extraction/tick 16S analysis and IGS nested PCR, respectively. Dr. Norbert Wolff from Infectolab Vet gave invaluable assistance in initializing tick collection. Wiktoria Fiedorowicz performed the gel extraction for tick 16S sequencing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Page, S., Daschkin, C., Anniko, S. et al. First report of Borrelia miyamotoi in an Ixodes ricinus tick in Augsburg, Germany. Exp Appl Acarol 74, 191–199 (2018). https://doi.org/10.1007/s10493-018-0220-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-018-0220-8