Abstract
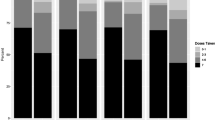
Few studies investigating daily oral preexposure prophylaxis (PrEP) focus on transgender persons. The Sustainable Health Center Implementation PrEP Pilot (SHIPP) Study included a large observational cohort of transgender persons with implications for PrEP in the United States. We examined data from SHIPP’s observational cohort and its Medication Adherence Substudy (MAS) to understand adherence among transgender participants in Chicago, IL. We assessed adherence by the proportion of days covered (PDC) for PrEP medication prescriptions, self-reported interview data, and concentrations of intracellular tenofovir diphosphate (TFV-DP) in dried blood spot (DBS) samples. Between 2014 and 2018, there were 510 transgender participants, 349 (68.4%) transgender women and 152 (29.8%) transgender men. Forty-five of these participants were enrolled in the MAS, 31 (68.9%) transgender women and 9 (20.0%) transgender men. By the 3-month follow up, 100% of MAS participants who completed an interview reported taking 4 or more doses of PrEP in the previous week. At 6, 9, and 12 months, taking 4 or more doses in the past week was reported by 81.0%, 94.1%, and 83.3% of participants, respectively. Results from TFV-DP DBS indicated that fewer participants reached the same level of adherence (4 or more doses/week) at clinical visits compared to self-report and even fewer participants reached this level of adherence based on the calculated PDC. Among participants who remained on PrEP throughout the study, DBS adherence levels declined after the first three months. There remains a critical need to develop strategies to address barriers and interventions that support PrEP adherence among transgender people.

Similar content being viewed by others
Data Availability
The data that support the findings of this study are not openly available due to the sensitivity of the clinical data.
Code Availability
Not applicable.
References
Centers for Disease Control and Prevention. HIV and transgender people. https://www.cdc.gov/hiv/pdf/group/gender/transgender/cdc-hiv-transgender-factsheet.pdf. Published April 2021. [Accessed July 6, 2021].
Centers for Disease Control and Prevention. Surveillance Report HIV. 2019; vol.32. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2021. [Accessed July 6, 2021].
Becasen JS, Denard CL, Mullins MM, Higa DH, Sipe TA. Estimating the prevalence of HIV and sexual behaviors among the US transgender population: A systematic review and meta-analysis, 2006–2017. AJPH. 2019;109:e1–8.
Centers for Disease Control and Prevention. US Public Health Service: preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: a clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Published March 2018. [Accessed July 6, 2021].
Escudero DJ, Kerr T, Operario D, Socias ME, Sued O, Marshall BD. Inclusion of trans women in pre-exposure prophylaxis trials: a review. AIDS Care. 2015;27(5):637–41.
Liu A, Cohen S, Follansbee S, Cohan D, Weber S, Sachdev D, et al. Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Med. 2014;11(3):e1001613.
Rael CT, Martinez M, Giguere R, Bockting W, MacCrate C, Mellman W, et al. Barriers and facilitators to oral PrEP use among transgender women in New York City. AIDS Behav. 2018;22(11):3627–36.
Landovitz RJ, Donnell D, Clement ME, Hanscom B, Cottle L, Coelho L. et. al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595–608.
Deutsch MB, Glidden DV, Sevelius J, Keatley J, McMahan V, Guanira J, et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV. 2015;2:e512–9.
HIV Prevention Trials Network. https://www.hptn.org/research/studies. Published June 2021. [Accessed July 6, 2021].
Grant RM, Sevelius JM, Guanire JV, Aguilar JV, Chariyalertsak S, Deutsch MB. Transgender women in clinical trials of pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):226–9.
Pacífico de Carvalho N, Mendicino CCP, Cândido RCF, Alecrim DJD, Menezes de Pádua CA. HIV pre-exposure prophylaxis (PrEP) awareness and acceptability among trans women: a review. AIDS Care. 2019;31(10):1234–40.
Nau DP. Proportion of days covered (PDC) as a preferred method of measuring medication adherence. Pharmacy Quality Alliance; 2012.
Pyra M, Rusie L, Castro M, Keglovitz Baker K, McNulty M, Bohm N, et al. A taxonomy of pragmatic measures of HIV preexposure prophylaxis use. AIDS. 2020;34(13):1951–7.
Sevelius JM, Keatley J, Calma N, Arnold E. ‘I am not a man’: trans-specific barriers and facilitators to PrEP acceptability among transgender women. Glob Public Health. 2016:1–16.
Wood SM, Lee S, Barg FK, Castillo M, Dowshen N. Young transgender women’s attitudes toward HIV pre-exposure prophylaxis. J Adolesc Health. 2017;60(5):549–55.
Reisner SL, Radix A, Deutsch MB. Integrated and gender-affirming transgender clinical care and research. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):235–42.
Mayer KH, Grinsztejn B, El-Sadr WM. Transgender people and HIV prevention: what we know and what we need to know, a call to action. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):207–9.
Smith DK, Rawlings MK, Glick N, Mena L, Coleman M, Houlberg M, et al. Adherence to daily oral TDF/FTC for prep in community health center populations: the sustainable health center implementation prep pilot (SHIPP) study. AIDS Behav. https://doi.org/10.1007/s10461-021-03388-5. Published online August 2021.
Hiransuthikul A, Janamnuaysook R, Himmad K, Kerr SJ, Thammajaruk N, Pankam T, et al. Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. J Int AIDS Soc. 2019;22(7):e25338.
Shieh E, Marzinke MA, Fuchs EJ, Hamlin A, Bakshi R, Aung W, et al. Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking estrogen when compared to cisgender men. J Int AIDS Soc. 2019;22(11):e25405.
Yager JL, Anderson PL. Pharmacology and drug interactions with HIV prep in transgender persons receiving affirming hormone therapy. Expert Opin Drug Metab Toxicol. 2020;16(6):463–74.
Grant RM, Pellegrini M, Defechereuz PA, Anderson PL, Yu M, Glidden DV. et.al. Sex hormone therapy and tenofovir diphosphate concentration in dried blood spots: primary results of the iBrEATHe study. Clin Infect Dis. 2020; n. pag.
Sevelius JM, Deutsch MB, Grant R. The future of PrEP among transgender women; the critical role of gender affirmation in research and clinical practices. J Int AIDS Soc. 2016;19(Suppl 6):21105.
Marcus JL, Buisker T, Horvath T, Amico KR, Fuchs JD, Buchbinder SP, et al. Helping our patients take HIV pre-exposure prophylaxis (PrEP): a systematic review of adherence interventions. HIV Med. 2014;15(7):385–95.
Radix AE, Lelutiu-Weinberger C, Gamarel KE. Satisfaction and healthcare utilization of transgender and gender non-conforming individuals in NYC: a community-based participatory study. LGBT Health. 2014;1(4):302–8.
Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Curr Opin HIV AIDS. 2016;11(1):10–7.
Fauci AS, Redfield RR, Sigounas G. et. al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844–5.
Funding
The SHIPP study was funded by the National Center for HIV, STD, Viral Hepatitis and TB Prevention at the Centers for Disease Control and Prevention and by donations to the CDC Foundation.
Author information
Authors and Affiliations
Contributions
Study conceptualization and design were performed by Ashley Townes and Dawn K. Smith. Data analyses were performed by Aruna Surendera Babu, Tiffany Williams, and Jeffrey Wiener. The first draft of the manuscript was written by Ashley Townes, Maria Pyra, and Dawn K. Smith. All authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
CDC Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This analysis was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention.
Financial Disclosure
No financial relationships relevant to this article to disclose.
Conflict of Interest
The authors declare that they have no relevant financial or nonfinancial conflicts of interest to disclose.
Ethics Approval
The SHIPP Study received human subjects research review and approval by the CDC IRB and the IRB of the City of Philadelphia IRB and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to Participate
All participants in the SHIPP medication adherence substudy (MAS) provided written informed consent for data and specimen collection and for publication of anonymized data.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Townes, A., Pyra, M., Smith, D.K. et al. PrEP Use and Adherence among Transgender Persons in Chicago, IL (SHIPP Study, 2014–2018, USA). AIDS Behav 27, 901–908 (2023). https://doi.org/10.1007/s10461-022-03826-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-022-03826-y