Abstract
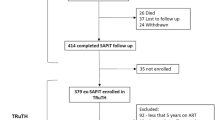
In the CHAP randomized placebo-controlled trial of cotrimoxazole prophylaxis in HIV-infected Zambian children conducted between 2001 and 2003, cotrimoxazole was associated with significant mortality reductions. In a secondary analysis we used Cox regression models to estimate the association between adherence measured by bottle weights and caregiver report and subsequent mortality in children surviving >28 days (n = 496, 153 deaths). Adherence was high and similar in both cotrimoxazole and placebo groups; adherence from bottle weights was 100% at 71% of visits, while caregivers reported 100% adherence at 79% of visits. Every 10% lower adherence to cotrimoxazole or placebo measured by bottle weights was associated with a 10–11% increase in mortality risk. Effects remained after adjustment for baseline predictors of survival and for current and recent change in primary caregiver. Caregiver-reported adherence was not associated with survival. The association between bottle-weight adherence to placebo and survival is likely capturing unmeasured caregiver effects, whose identification will be essential for quantifying the impact of antiretroviral therapy (ART) adherence on clinical outcomes in children.

Similar content being viewed by others
References
Anglaret, X., Chene, G., Attia, A., Toure, S., Lafont, S., Combe, P., et al. (1999). Early chemoprophylaxis with trimethtoprim–sulphamethoxazole for HIV-1 infected adults in Abidjan, Cote d’Ivoire: A randomised trial. The Lancet, 353, 1463–1468.
Badri, M., Maartens, G., Wood, R., & Ehrlich, R. (1999). Co-trimoxazole in HIV-1 infection. The Lancet, 354, 334–335.
Bangsberg, D. R., Perry, S., Charlebois, E. D., Clark, R. A., Robertson, M., Zolopa, A. R., et al. (2001). Nonadherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS, 15, 1181–1183.
Bikaako-Kajura, W., Luyirika, E., Purcell, D. W., Downing, J., Kaharuza, F., Mermin, J., et al. (2006). Disclosure of HIV status and adherence to daily drug regimens among HIV-infected children in Uganda. AIDS & Behavior, 10, S85–S93.
Chintu, C., Bhat, G. J., Walker, A. S., Mulenga, V., Sinyinza, F., Lishimpi, K., et al. (2004). Cotrimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): A double-blind randomized placebo-controlled trial. The Lancet, 364, 1865–1871.
Eldred, L. J., Wu, A. W., Chaisson, R. E., & Moore, R. D. (1998). Adherence to antretroviral and pneumocystis prophylaxis in HIV disease. Journal of Acquired Immune Deficiency Syndromes & Human Retrovirology, 18, 117–125.
El-Sadr, W. M., Luskin-Hawk, R., Yurik, T. M., Walker, J., Abrams, D., John, S. L., et al. (1999). A randomized trial of daily and thrice-weekly trimethoprim–sulfamethoxazole for the prevention of Pneumocystis carinii pneumonia in human immunodeficiency virus-infected persons. Clinical Infectious Diseases, 29, 775–783.
Freeman, J. V., Cole, T. J., Chinn, S, Jones, P. R. M., White, E. M., & Preece, M. A. (1995). Cross sectional stature and weight reference curves for the UK, 1990. Archives of Disease in Childhood, 73, 17–24.
Gibb, D. M., Goodall, R. L., Giacomet, V., McGee, L., Compagnucci, A., & Lyall, H., Paediatric European Network for Treatment of AIDS Steering Committee. (2003). Adherence to prescribed antiretroviral therapy in human immunodeficiency virus-infected children in the PENTA 5 trial. Pediatric Infectious Disease Journal, 22, 56–62.
Grimwade, K., Sturm, A. W., Nunn, A. J., Mbatha, D., Zungu, D., & Gilks, C. F. (2005). Effectiveness of cotrimoxazole on mortality in adults with tuberculosis in rural South Africa. AIDS, 19, 163–168.
Hess, K. R. (1994). Assessing time-by-covariate interactions in proportional hazards regression models using cubic spline functions. Statistics in Medicine, 13, 1045–1062.
Jones, J. L., Hanson, D. L., Dworkin, M. S., Alderton, D. L., Fleming, P. L., Kaplan, J. E., et al. (1999). Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. Morbidity & Mortality Weekly Report. Surveillance Summaries, 48, 1–22.
Lee, J. Y., Kusek, J. W., Greene, P. G., Bernhard, S., Norris, K., Smith, D., et al. (1996). Assessing medication adherence by pill count and electronic monitoring in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. American Journal of Hypertension, 9, 719–725.
Liu, H., Golin, C. E., Miller, L. G., Hays, R. D., Beck, C. K., Sanandaji, S., et al. (2001). A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine, 134, 968–977. Erratum in: Annals of Internal Medicine, 136, 175, 2002.
Mannheimer, S., Friedland, G., Matts, J., Child, C., & Chesney, M. (2002). The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clinical Infectious Diseases, 34, 1115–1121.
Martin, S., Elliott-DeSorbo, D. K., Wolters, P. L., Toledo-Tamula, M. A., Roby, G., Zeichner, S., et al. (2007). Patient, caregiver and regimen characteristics associated with adherence to highly active antiretroviral therapy among HIV-infected children and adolescents. Pediatric Infectious Disease Journal, 26, 61–67.
Matsui, D., Hermann, C., Klein, J., Berkovitch, M., Olivieri, N., & Koren, G. (1994). Critical comparison of novel and existing methods of compliance assessment during a clinical trial of an oral iron chelator. Journal of Clinical Pharmacology, 34, 944–949.
McIntosh, N., Helms, P., & Smyth, R. (2003) Forfar and Arnneil’s textbook of paediatrics (6th ed., pp. 576–577). London: Churchill Livingstone.
McNabb, J. J., Nicolau, D. P., Stoner, J. A., & Ross, J. (2003). Patterns of adherence to antiretroviral medications: the value of electronic monitoring. AIDS, 17, 1763–1767.
Mermin, J., Lule, J., Ekwaru, J. P., Malamba, S., Downing, R., Ransom, R., et al. (2004). Effect of cotrimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. The Lancet, 364, 1428–1434.
Mills, E. J., Nachega, J. B., Bangsberg, D. R., Singh, S., Rachlis, B., Wu, P., et al. (2006). Adherence to HAART; a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Medicine, 3(11), e438.
Moss, A. R., Hahn, J. A., Perry, S., Charlebois, E. D., Guzman, D., Clark, R. A., et al. (2004). Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: A prospective study. Clinical Infectious Diseases, 39, 1190–1198.
Simpson, S. H., Eurich, D. T., Majumdar, S. R., Padwal, R. S., Tsuyuki, R. T., Varney, J., et al. (2006). A meta-analysis of the association between adherence to drug therapy and mortality. British Medical Journal, 333(7557):15.
Walker, A. S., Mulenga, V., Sinyinza, F., Lishimpi, K., Nunn, A., Chintu, C., et al. (2006). Determinants of survival without antiretroviral therapy after infancy in HIV-1-infected Zambian children in the CHAP trial. Journal of Acquired Immune Deficiency Syndromes, 42, 637–645.
Waterhouse, D. M., Calzone, K. A., Mele, C., & Brenner, D. E. (1993). Adherence to oral tamoxifen: A comparison of patient self-report, pill counts and microelectronic monitoring. Journal of Clinical Oncology, 11, 1189–1197.
Wiktor, S. Z., Sassan-Morroko, M., Grant, A. D., Abouya, L., Karon, J. M., Maurice, C., et al. (1999). Efficacy of trimethoprim–sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1 infected patients with tuberculosis in Abidjan, Cote d’Ivoire: A randomised controlled trial. The Lancet, 353, 1469–1475. Erratum in: The Lancet, 353, 2078, 1999.
Wood, E., Hogg, R. S., Yip, B., Moore, D., Harrigan, P. R., & Montaner, J. S. (2006). Impact of baseline viral load and adherence on survival of HIV-infected adults with baseline CD4 cell counts > or =200 cells/microl. AIDS, 20, 1117–1123.
World Health Organization. (2006). Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults in resource-limited settings: Recommendations for a public health approach. Retrieved from http://www.womenchildrenhiv.org. Accessed 31 Mar 2008.
Acknowledgements
We would like to thank the families and children enrolled in the CHAP trial. We also acknowledge Laura Farrelly, Nicola Kaganson and Patrick Phillips who organised adherence measurements in the trial and carried out initial exploratory analyses.
Sources of Support
The CHAP trial was funded by the Department for International Development, UK.
Cotrimoxazole and matching placebo suspension were provided by Interchem Ltd, Lusaka, and quality was tested at the Quality Control North West, Liverpool, UK.
The CHAP Trial Team
University Teaching Hospital, Lusaka, Zambia: Principal Investigators (Prof Chifumbe Chintu, Prof Ganapati Bhat), Project Management/Paediatricians (Dr Veronica Mulenga, Dr Kennedy Lishimpi, Dr Frederick Sinyinza, Dr Desire Kabamba), Microbiology (James Mwansa, Darlington Mwenya, Mr Mutela), Clinic Staff (Regina Chileshe, Catherine Kalengo, J Kaluwaji, Agness Simuchoba, Mrs Mutengo, Violet Bwalya, Mr Chitambala, Terence Chipoya, Betty Chanda), Data Management (Mwaka Choongo, Liffer Namakube, Paul Mutale), Virology (Gina Mulundu, Mrs Liwewe), Parasitology (Mr Mandanda), Pathology (Mr Mudenda).
Medical Research Council Clinical Trials Unit, London: Principal Investigators (Dr Di Gibb, Prof Andrew Nunn), Project/Data Management (Laura Farrelly, Nicola Kaganson, Dr Julia Abernethy, Dr Margaret Thomason, Alexander Ferrier), Statisticians (Prof Andrew Nunn, Dr Sarah Walker, Dr Deborah Ford).
Royal Free Hospital, London: Microbiology (Prof Stephen Gillespie, Sushma Patel, Claire Ling).
University College London: Principal Investigators (Prof Ali Zumla).
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors are on behalf of the Children with HIV Antibiotic Prophylaxis Trial (CHAP Trial).
Rights and permissions
About this article
Cite this article
Walker, A.S., Ford, D., Mulenga, V. et al. Adherence to Both Cotrimoxazole and Placebo is Associated with Improved Survival Among HIV-Infected Zambian Children. AIDS Behav 13, 33–41 (2009). https://doi.org/10.1007/s10461-008-9382-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-008-9382-4