Abstract
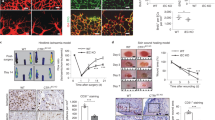
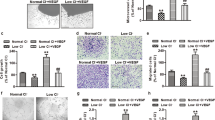
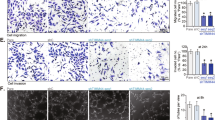
VEGFR2 signaling in endothelial cells (ECs) is regulated by reactive oxygen species (ROS) derived from NADPH oxidases (NOXs) and mitochondria, which plays an important role in postnatal angiogenesis. However, it remains unclear how highly diffusible ROS signal enhances VEGFR2 signaling and reparative angiogenesis. Protein disulfide isomerase A1 (PDIA1) functions as an oxidoreductase depending on the redox environment. We hypothesized that PDIA1 functions as a redox sensor to enhance angiogenesis. Here we showed that PDIA1 co-immunoprecipitated with VEGFR2 or colocalized with either VEGFR2 or an early endosome marker Rab5 at the perinuclear region upon stimulation of human ECs with VEGF. PDIA1 silencing significantly reduced VEGF-induced EC migration, proliferation and spheroid sprouting via inhibiting VEGFR2 signaling. Mechanistically, VEGF stimulation rapidly increased Cys-OH formation of PDIA1 via the NOX4–mitochondrial ROS axis. Overexpression of “redox-dead” mutant PDIA1 with replacement of the active four Cys residues with Ser significantly inhibited VEGF-induced PDIA1–CysOH formation and angiogenic responses via reducing VEGFR2 phosphorylation. Pdia1+/− mice showed impaired angiogenesis in developmental retina and Matrigel plug models as well as ex vivo aortic ring sprouting model. Study using hindlimb ischemia model revealed that PDIA1 expression was markedly increased in angiogenic ECs of ischemic muscles, and that ischemia-induced limb perfusion recovery and neovascularization were impaired in EC-specific Pdia1 conditional knockout mice. These results suggest that PDIA1 can sense VEGF-induced H2O2 signal via CysOH formation to promote VEGFR2 signaling and angiogenesis in ECs, thereby enhancing postnatal angiogenesis. The oxidized PDIA1 is a potential therapeutic target for treatment of ischemic vascular diseases.








Similar content being viewed by others
Data availability
The authors declare that all supporting data are available within the article (and its data supplement).
References
Ushio-Fukai M (2006) Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res 71(2):226–235. https://doi.org/10.1016/j.cardiores.2006.04.015
Ushio-Fukai M (2007) VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 9(6):731–739. https://doi.org/10.1089/ars.2007.1556
Ushio-Fukai M, Urao N (2009) Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal 11(10):2517–2533. https://doi.org/10.1089/ARS.2009.2582
Fukai T, Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15(6):1583–1606. https://doi.org/10.1089/ars.2011.3999
Lee SR, Kwon KS, Kim SR, Rhee SG (1998) Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 273(25):15366–15372. https://doi.org/10.1074/jbc.273.25.15366
Simons M (2012) An inside view: VEGF receptor trafficking and signaling. Physiology (Bethesda) 27(4):213–222. https://doi.org/10.1152/physiol.00016.2012
Eichmann A, Simons M (2012) VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol 24(2):188–193. https://doi.org/10.1016/j.ceb.2012.02.002
Fukai T, Ushio-Fukai M (2020) Cross-talk between NADPH oxidase and mitochondria: role in ROS signaling and angiogenesis. Cells. https://doi.org/10.3390/cells9081849
Kim YM, Kim SJ, Tatsunami R, Yamamura H, Fukai T, Ushio-Fukai M (2017) ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am J Physiol Cell Physiol 312(6):C749–C764. https://doi.org/10.1152/ajpcell.00346.2016
Poole LB, Karplus PA, Claiborne A (2004) Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol 44:325–347. https://doi.org/10.1146/annurev.pharmtox.44.101802.121735
Ushio-Fukai M (2006) Localizing NADPH oxidase-derived ROS. Sci STKE. https://doi.org/10.1126/stke.3492006re8
Charles RL, Schroder E, May G, Free P, Gaffney PR, Wait R, Begum S, Heads RJ, Eaton P (2007) Protein sulfenation as a redox sensor: proteomics studies using a novel biotinylated dimedone analogue. Mol Cell Proteomics 6(9):1473–1484. https://doi.org/10.1074/mcp.M700065-MCP200
Poole LB, Nelson KJ (2008) Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol 12(1):18–24. https://doi.org/10.1016/j.cbpa.2008.01.021
Benham AM (2012) The protein disulfide isomerase family: key players in health and disease. Antioxid Redox Signal 16(8):781–789. https://doi.org/10.1089/ars.2011.4439
Laurindo FR, Pescatore LA, Fernandes Dde C (2012) Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radical Biol Med 52(9):1954–1969. https://doi.org/10.1016/j.freeradbiomed.2012.02.037
Cremers CM, Jakob U (2013) Oxidant sensing by reversible disulfide bond formation. J Biol Chem 288(37):26489–26496. https://doi.org/10.1074/jbc.R113.462929
Xiong B, Jha V, Min JK, Cho J (2020) Protein disulfide isomerase in cardiovascular disease. Exp Mol Med 52(3):390–399. https://doi.org/10.1038/s12276-020-0401-5
Kim YM, Youn SW, Sudhahar V, Das A, Chandhri R, Cuervo Grajal H, Kweon J, Leanhart S, He L, Toth PT, Kitajewski J, Rehman J, Yoon Y, Cho J, Fukai T, Ushio-Fukai M (2018) Redox regulation of mitochondrial fission protein Drp1 by protein disulfide isomerase limits endothelial senescence. Cell Rep 23(12):3565–3578. https://doi.org/10.1016/j.celrep.2018.05.054
Laurindo FR, Fernandes DC, Amanso AM, Lopes LR, Santos CX (2008) Novel role of protein disulfide isomerase in the regulation of NADPH oxidase activity: pathophysiological implications in vascular diseases. Antioxid Redox Signal 10(6):1101–1113. https://doi.org/10.1089/ars.2007.2011
Hahm E, Li J, Kim K, Huh S, Rogelj S, Cho J (2013) Extracellular protein disulfide isomerase regulates ligand-binding activity of alphaMbeta2 integrin and neutrophil recruitment during vascular inflammation. Blood 121(19):3789–3800. https://doi.org/10.1182/blood-2012-11-467985
Zhou J, Wu Y, Wang L, Rauova L, Hayes VM, Poncz M, Essex DW (2015) The C-terminal CGHC motif of protein disulfide isomerase supports thrombosis. J Clin Invest 125(12):4391–4406. https://doi.org/10.1172/JCI80319
Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA (2009) Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457(7231):887–891. https://doi.org/10.1038/nature07619
Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K (2011) Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc 7(1):89–104. https://doi.org/10.1038/nprot.2011.435
Chen GF, Sudhahar V, Youn SW, Das A, Cho J, Kamiya T, Urao N, McKinney RD, Surenkhuu B, Hamakubo T, Iwanari H, Li S, Christman JW, Shantikumar S, Angelini GD, Emanueli C, Ushio-Fukai M, Fukai T (2015) Copper transport protein antioxidant-1 promotes inflammatory neovascularization via chaperone and transcription factor function. Sci Rep 5:14780. https://doi.org/10.1038/srep14780
Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW (2005) Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111(18):2347–2355. https://doi.org/10.1161/01.CIR.0000164261.62586.14
Urao N, Sudhahar V, Kim SJ, Chen GF, McKinney RD, Kojda G, Fukai T, Ushio-Fukai M (2013) Critical role of endothelial hydrogen peroxide in post-ischemic neovascularization. PLoS ONE 8(3):e57618. https://doi.org/10.1371/journal.pone.0057618
Das A, Sudhahar V, Chen GF, Kim HW, Youn SW, Finney L, Vogt S, Yang J, Kweon J, Surenkhuu B, Ushio-Fukai M, Fukai T (2016) Endothelial antioxidant-1: a key mediator of copper-dependent wound healing in vivo. Sci Rep 6:33783. https://doi.org/10.1038/srep33783
Chabot S, Jabrane-Ferrat N, Bigot K, Tabiasco J, Provost A, Golzio M, Noman MZ, Giustiniani J, Bellard E, Brayer S, Aguerre-Girr M, Meggetto F, Giuriato S, Malecaze F, Galiacy S, Jais JP, Chose O, Kadouche J, Chouaib S, Teissie J, Abitbol M, Bensussan A, Le Bouteiller P (2011) A novel antiangiogenic and vascular normalization therapy targeted against human CD160 receptor. J Exp Med 208(5):973–986. https://doi.org/10.1084/jem.20100810
Oshikawa J, Kim SJ, Furuta E, Caliceti C, Chen GF, McKinney RD, Kuhr F, Levitan I, Fukai T, Ushio-Fukai M (2012) Novel role of p66Shc in ROS-dependent VEGF signaling and angiogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 302(3):H724-732. https://doi.org/10.1152/ajpheart.00739.2011
Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, McKinney R, Poole LB, Fukai T, Ushio-Fukai M (2010) Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS ONE 5(4):e10189. https://doi.org/10.1371/journal.pone.0010189
Wang S, Amato KR, Song W, Youngblood V, Lee K, Boothby M, Brantley-Sieders DM, Chen J (2015) Regulation of endothelial cell proliferation and vascular assembly through distinct mTORC2 signaling pathways. Mol Cell Biol 35(7):1299–1313. https://doi.org/10.1128/MCB.00306-14
Ash D, Sudhahar V, Youn SW, Okur MN, Das A, O’Bryan JP, McMenamin M, Hou Y, Kaplan JH, Fukai T, Ushio-Fukai M (2021) The P-type ATPase transporter ATP7A promotes angiogenesis by limiting autophagic degradation of VEGFR2. Nat Commun 12(1):3091. https://doi.org/10.1038/s41467-021-23408-1
Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M (2008) Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103(2):212–220. https://doi.org/10.1161/CIRCRESAHA.108.176230
Simons M, Gordon E, Claesson-Welsh L (2016) Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 17(10):611–625. https://doi.org/10.1038/nrm.2016.87
Freedman RB, Gane PJ, Hawkins HC, Hlodan R, McLaughlin SH, Parry JW (1998) Experimental and theoretical analyses of the domain architecture of mammalian protein disulphide-isomerase. Biol Chem 379(3):321–328. https://doi.org/10.1515/bchm.1998.379.3.321
Alanen HI, Salo KE, Pekkala M, Siekkinen HM, Pirneskoski A, Ruddock LW (2003) Defining the domain boundaries of the human protein disulfide isomerases. Antioxid Redox Signal 5(4):367–374. https://doi.org/10.1089/152308603768295096
Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M (2008) Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res 102(10):1182–1191. https://doi.org/10.1161/CIRCRESAHA.107.167080
Lanahan AA, Lech D, Dubrac A, Zhang J, Zhuang ZW, Eichmann A, Simons M (2014) PTP1b is a physiologic regulator of vascular endothelial growth factor signaling in endothelial cells. Circulation 130(11):902–909. https://doi.org/10.1161/CIRCULATIONAHA.114.009683
Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D (2003) Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423(6941):769–773. https://doi.org/10.1038/nature01680
Londhe AD, Bergeron A, Curley SM, Zhang F, Rivera KD, Kannan A, Coulis G, Rizvi SHM, Kim SJ, Pappin DJ, Tonks NK, Linhardt RJ, Boivin B (2020) Regulation of PTP1B activation through disruption of redox-complex formation. Nat Chem Biol 16(2):122–125. https://doi.org/10.1038/s41589-019-0433-0
Tian F, Zhou X, Wikstrom J, Karlsson H, Sjoland H, Gan LM, Boren J, Akyurek LM (2009) Protein disulfide isomerase increases in myocardial endothelial cells in mice exposed to chronic hypoxia: a stimulatory role in angiogenesis. Am J Physiol Heart Circ Physiol 297(3):H1078-1086. https://doi.org/10.1152/ajpheart.00937.2008
Li J, Kim K, Jeong SY, Chiu J, Xiong B, Petukhov PA, Dai X, Li X, Andrews RK, Du X, Hogg PJ, Cho J (2019) Platelet protein disulfide isomerase promotes glycoprotein ibalpha-mediated platelet-neutrophil interactions under thromboinflammatory conditions. Circulation 139(10):1300–1319. https://doi.org/10.1161/CIRCULATIONAHA.118.036323
Camargo LL, Babelova A, Mieth A, Weigert A, Mooz J, Rajalingam K, Heide H, Wittig I, Lopes LR, Brandes RP (2013) Endo-PDI is required for TNFalpha-induced angiogenesis. Free Radical Biol Med 65:1398–1407. https://doi.org/10.1016/j.freeradbiomed.2013.09.028
Kang DH, Lee DJ, Lee KW, Park YS, Lee JY, Lee SH, Koh YJ, Koh GY, Choi C, Yu DY, Kim J, Kang SW (2011) Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Mol Cell 44(4):545–558. https://doi.org/10.1016/j.molcel.2011.08.040
Kenche H, Ye ZW, Vedagiri K, Richards DM, Gao XH, Tew KD, Townsend DM, Blumental-Perry A (2016) Adverse outcomes associated with cigarette smoke radicals related to damage to protein-disulfide isomerase. J Biol Chem 291(9):4763–4778. https://doi.org/10.1074/jbc.M115.712331
Townsend DM, Manevich Y, He L, Xiong Y, Bowers RR Jr, Hutchens S, Tew KD (2009) Nitrosative stress-induced s-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res 69(19):7626–7634. https://doi.org/10.1158/0008-5472.CAN-09-0493
Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA (2006) S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441(7092):513–517. https://doi.org/10.1038/nature04782
Wroblewski VJ, Masnyk M, Khambatta SS, Becker GW (1992) Mechanisms involved in degradation of human insulin by cytosolic fractions of human, monkey, and rat liver. Diabetes 41(4):539–547. https://doi.org/10.2337/diab.41.4.539
Turano C, Coppari S, Altieri F, Ferraro A (2002) Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol 193(2):154–163. https://doi.org/10.1002/jcp.10172
Parakh S, Atkin JD (2015) Novel roles for protein disulphide isomerase in disease states: a double edged sword? Front Cell Dev Biol 3:30. https://doi.org/10.3389/fcell.2015.00030
El Hindy M, Hezwani M, Corry D, Hull J, El Amraoui F, Harris M, Lee C, Forshaw T, Wilson A, Mansbridge A, Hassler M, Patel VB, Kehoe PG, Love S, Conway ME (2014) The branched-chain aminotransferase proteins: novel redox chaperones for protein disulfide isomerase–implications in Alzheimer’s disease. Antioxid Redox Signal 20(16):2497–2513. https://doi.org/10.1089/ars.2012.4869
Soares Moretti AI, Martins Laurindo FR (2017) Protein disulfide isomerases: redox connections in and out of the endoplasmic reticulum. Arch Biochem Biophys 617:106–119. https://doi.org/10.1016/j.abb.2016.11.007
Raiborg C, Wenzel EM, Stenmark H (2015) ER-endosome contact sites: molecular compositions and functions. EMBO J 34(14):1848–1858. https://doi.org/10.15252/embj.201591481
Aoyama-Ishiwatari S, Hirabayashi Y (2021) Endoplasmic reticulum-mitochondria contact sites-emerging intracellular signaling hubs. Front Cell Dev Biol 9:653828. https://doi.org/10.3389/fcell.2021.653828
Boivin B, Yang M, Tonks NK (2010) Targeting the reversibly oxidized protein tyrosine phosphatase superfamily. Sci Signal. https://doi.org/10.1126/scisignal.3137pl2
Frangioni JV, Beahm PH, Shifrin V, Jost CA, Neel BG (1992) The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell 68(3):545–560. https://doi.org/10.1016/0092-8674(92)90190-n
Acknowledgements
We would like to thank Dr. Henar Cyuervo Grajal at University of Illinois at Chicago for assisting retinal angiogenesis in initial study.
Funding
This work was supported by National Institute of Health grants: R01HL160014, R01HL135584 (to M.U.-F.), R01HL147550 (to M.U.-F., T.F), R01HL133613, R01HL116976 (to T.F., M.U.-F.), R01HL070187 (to T.F.), R01EY011766, R01EY030500, R21EY032265 (R.B.C.), Veterans Administration Merit Review Award 2I01BX001232 (to T.F.), 101BX001233 (R.B.C.), R01HL118526 (D.W.E.). The VA Career Scientist Award (IK6BX005228) (to R.B.C.). R.B. Caldwell is the recipient of a Research Career Scientist Award from the Department of Veterans Affairs. The contents do not represent the views of the Department of Veterans Affairs or the United States Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
MU-F, TF, SN, Y-MK, designed the study; SN, Y-MK, DA, VS, SWY, YH, AD, EV, TAR performed experiments. MM, HB, performed mouse genotyping. MU-F, TF, SN and Y-MK, DA, AD, analyzed data. VS, RC, DWE, JC, discussed data and provided inputs and reagents. MU-F, TF, SN, Y-MK, wrote the manuscript. RC, DWE, JC. edited the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
The animal protocols used in this study were approved by the institutional Animal Care Committee and institutional Biosafety Committee of University of Illinois at Chicago and Augusta University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagarkoti, S., Kim, YM., Ash, D. et al. Protein disulfide isomerase A1 as a novel redox sensor in VEGFR2 signaling and angiogenesis. Angiogenesis 26, 77–96 (2023). https://doi.org/10.1007/s10456-022-09852-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-022-09852-7