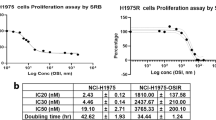
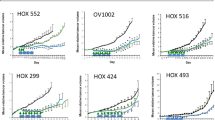
Abstract
An alternative or follow-up adjunct to conventional maximum tolerated dose (MTD) chemotherapy now in advanced phase III clinical trial assessment is metronomic chemotherapy—the close regular administration of low doses of drug with no prolonged breaks. A number of preclinical studies have shown metronomic chemotherapy can cause long term survival of mice with advanced cancer, including metastatic disease, in the absence of overt toxicity, especially when combined with targeted antiangiogenic drugs. However, similar to MTD chemotherapy acquired resistance eventually develops, the basis of which is unknown. Using a preclinical model of advanced human ovarian (SKOV-3-13) cancer in SCID mice, we show that acquired resistance can develop after terminating prolonged (over 3 months) successful therapy utilizing daily oral metronomic topotecan plus pazopanib, an oral antiangiogenic tyrosine kinase inhibitor (TKI). Two resistant sublines were isolated from a single mouse, one from a solid tumor (called KH092-7SD, referred to as 7SD) and another from ascites tumor cells (called KH092-7AS, referred to as 7AS). Using these sublines we show acquired resistance to the combination treatment is due to tumor cell alterations that confer relative refractoriness to topotecan. The resistant phenotype is heritable, associated with reduced cellular uptake of topotecan and could not be reversed by switching to MTD topotecan or to another topoisomerase-1 inhibitor, CPT-11, given either in a metronomic or MTD manner nor switching to another antiangiogenic drug, e.g. the anti-VEGFR-2 antibody, DC101, or another TKI, sunitinib. Thus, in this case cross resistance seems to exist between MTD and metronomic topotecan, the basis of which is unknown. However, gene expression profiling revealed several potential genes that are stably upregulated in the resistant lines, that previously have been implicated in resistance to various chemotherapy drugs, and which, therefore, may contribute to the drug resistant phenotype.









Similar content being viewed by others
References
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4(6):423–436
Pasquier E, Kavallaris M, Andre N (2010) Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol 7(8):455–465
Rapisarda A et al (2004) Schedule-dependent inhibition of hypoxia-inducible factor-1alpha protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Res 64(19):6845–6848
Lee K et al (2009) Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci USA 106(7):2353–2358
Folkins C et al (2009) Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res 69(18):7243–7251
Martin-Padura I et al (2012) Residual dormant cancer stem-cell foci are responsible for tumor relapse after antiangiogenic metronomic therapy in hepatocellular carcinoma xenografts. Lab Invest 92(7):952–966
Browder T et al (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60(7):1878–1886
Klement G et al (2000) Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 105(8):R15–R24
Daenen LG et al (2009) Low-dose metronomic cyclophosphamide combined with vascular disrupting therapy induces potent antitumor activity in preclinical human tumor xenograft models. Mol Cancer Ther 8(10):2872–2881
Shaked Y et al (2005) Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood 106(9):3058–3061
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3(10):721–732
Munoz R et al (2006) Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res 66(7):3386–3391
Hashimoto K et al (2010) Potent preclinical impact of metronomic low-dose oral topotecan combined with the antiangiogenic drug pazopanib for the treatment of ovarian cancer. Mol Cancer Ther 9(4):996–1006
Merritt WM et al (2009) Anti-angiogenic properties of metronomic topotecan in ovarian carcinoma. Cancer Biol Ther 8(16):1596–1603
Emmenegger U et al (2011) Tumors that acquire resistance to low-dose metronomic cyclophosphamide retain sensitivity to maximum tolerated dose cyclophosphamide. Neoplasia 13(1):40–48
Kerbel RS (2012) Strategies for improving the clinical benefit of antiangiogenic drug based therapies for breast cancer. J Mammary Gland Biol Neoplasia 17(3–4):229–239
Bocci G et al (2008) Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br J Cancer 98(10):1619–1629
Shen J et al (2009) Compartment-specific roles of ATP-binding cassette transporters define differential topotecan distribution in brain parenchyma and cerebrospinal fluid. Cancer Res 69(14):5885–5892
Hubbard KE et al (2009) Application of a highly specific and sensitive fluorescent HPLC method for topotecan lactone in whole blood. Biomed Chromatogr 23(7):707–713
Folkins C et al (2007) Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res 67(8):3560–3564
Mizuno H, Kitada K, Nakai K, Sarai A (2009) PrognoScan: a new database for meta-analysis of the prognostic value of genes. BMC Med Genomics 2:18
McFadyen MC et al (2001) Cytochrome P450 CYP1B1 over-expression in primary and metastatic ovarian cancer. Br J Cancer 85(2):242–246
Garcia AA et al (2008) Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol 26(1):76–82
Dellapasqua S et al (2008) Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol 26(30):4899–4905
Bottini A et al (2006) Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol 24(22):3623–3628
Koopman M et al (2013) Maintenance treatment with capecitabine and bevacizumab versus observation after induction treatment with chemotherapy and bevacizumab in metastatic colorectal cancer (mCRC): the phase III CAIRO3 study of the Dutch Colorectal Cancer Group (DCCG). J Clin Oncol 31:Abstr 3502
Miklos GL (2012) Bevacizumab in neoadjuvant treatment for breast cancer. N Engl J Med 366(17):1638 (author reply 1639–40)
Montero AJ, Vogel C (2012) Fighting fire with fire: rekindling the bevacizumab debate. N Engl J Med 366(4):374–375
Dawood S et al (2012) The use of bevacizumab among women with metastatic breast cancer: a survey on clinical practice and the ongoing controversy. Cancer 118(11):2780–2786
Aogi K et al (2011) First-line bevacizumab in combination with weekly paclitaxel for metastatic breast cancer: efficacy and safety results from a large, open-label, single-arm Japanese study. Breast Cancer Res Treat 129(3):829–838
Thomssen C et al (2012) First-line bevacizumab-containing therapy for triple-negative breast cancer: analysis of 585 patients treated in the ATHENA study. Oncology 82(4):218–227
Smith I et al (2011) Final overall survival results and effect of prolonged (≥1 year) first-line bevacizumab-containing therapy for metastatic breast cancer in the ATHENA trial. Breast Cancer Res Treat 130(1):133–143
Barrios CH et al (2010) Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat 121(1):121–131
Bergh J, Greil R, Voytko N (2011) Sunitinib (SU) in combination with docetaxel (D) versus D alone for the first-line treatment of advanced breast cancer (ABC). J Clin Oncol 28:LBA 1010
Merritt WM et al (2010) Bridging the gap between cytotoxic and biologic therapy with metronomic topotecan and pazopanib in ovarian cancer. Mol Cancer Ther 9(4):985–995
Kumar S et al (2011) Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin Cancer Res 17(17):5656–5667
Hackl C et al (2013) Metronomic oral topotecan prolongs survival and reduces liver metastasis in improved preclinical orthotopic and adjuvant therapy colon cancer models. Gut 62(2):259–271
Goh F, Sambanis A (2011) In vivo noninvasive monitoring of dissolved oxygen concentration within an implanted tissue-engineered pancreatic construct. Tissue Eng Part C Methods 17(9):887–894
Kato H et al (2004) A randomized trial of adjuvant chemotherapy with Uracil–Tegafur for adenocarcinoma of the lung. New Engl J Med 350:1713–1721
Garcia et al (2008) Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and princess Margaret hospital phase II consortia. J Clin Oncol 26:76–82
Dellapasqua et al (2008) Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol 26:4899–4905
Berendsen HH et al (1988) Characterization of three small cell lung cancer cell lines established from one patient during longitudinal follow-up. Cancer Res 48:6891–6899
Kuczynski et al (2013) Drug rechallenge and treatment beyond progression in oncology: examples, mechanisms and implications of reversible drug resistance. Nature Rev Clin Oncol 10:571–587
Rochat B et al (2001) Human CYP1B1 and anticancer agent metabolism: mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther 296(2):537–541
Wittig R et al (2002) Candidate genes for cross-resistance against DNA-damaging drugs. Cancer Res 62(22):6698–6705
Oshita SE et al (2010) The small heat shock protein HspB2 is a novel anti-apoptotic protein that inhibits apical caspase activation in the extrinsic apoptotic pathway. Breast Cancer Res Treat 124(2):307–315
Langbein S et al (2006) Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer 94(4):578–585
Acknowledgments
This work was supported by grants to RSK from the Canadian Institutes of Health Research (CIHR) and the National Institutes of Health (NIH), USA (CA-41233) and the Canadian Breast Cancer Foundation (CBCF). RSK is a Canada Research Chair in Tumor Biology, Angiogenesis, and Antiangiogenic Therapy. M.L. Jaramillo, C. Collins, M. Banville and M.D. O'Connor-McCourt were supported by the NRC Genome Health Initiative Cancer Program (NRC publication number NRC5322).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cruz-Muñoz, W., Di Desidero, T., Man, S. et al. Analysis of acquired resistance to metronomic oral topotecan chemotherapy plus pazopanib after prolonged preclinical potent responsiveness in advanced ovarian cancer. Angiogenesis 17, 661–673 (2014). https://doi.org/10.1007/s10456-014-9422-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-014-9422-9