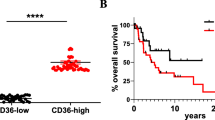
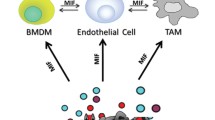
Abstract
The angiogenic potential of solid tumors, or the ability to initiate neovasculature development from pre-existing host vessels, is facilitated by soluble factors secreted by tumor cells and involves breaching of extracellular matrix barriers, endothelial cell (EC) proliferation, migration and reassembly. We evaluated the angiogenic potential of human melanoma cell lines differing in their degree of aggressiveness, based on their ability to regulate directionally persistent EC migration. We observed that conditioned-medium (CM) of the aggressive melanoma cell line BLM induced a high effective migratory response in ECs, while CMs of Mel57 and 1F6 had an inhibitory effect. Further, the melanoma cell lines exhibited a varied expression profile of tissue inhibitor of metalloproteinase-3 (TIMP3), detectable in the CM. TIMP3 expression inversely correlated with aggressiveness of the melanoma cell line, and ability of the respective CMs to induce directed EC migration. Interestingly, TIMP3 expression was found to be silenced in the BLM cell line, concurrent with its role as a tumor suppressor. Treatment with recombinant human TIMP3 and CM of modified, TIMP3 expressing, BLM cells mitigated directional EC migration, while CM of TIMP3 silenced 1F6 cells induced directed EC migration. The functional implication of TIMP3 expression on tumor growth and angiogenic potential in melanoma was evaluated in vivo. We observed that TIMP3 expression reduced tumor growth, angiogenesis and macrophage infiltration of BLM tumors while silencing TIMP3 increased tumor growth and angiogenesis of 1F6 tumors. Taken together, our results demonstrate that TIMP3 expression correlates with inhibition of directionally persistent EC migration and adversely affects the angiogenic potential and growth of melanomas.








Similar content being viewed by others
References
Mahabeleshwar GH, Byzova TV (2007) Angiogenesis in melanoma. Semin Oncol 34(6):555–565
Kalluri R (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3(6):422–433
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1(1):27–31
Folkman J (1995) Seminars in medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 333(26):1757–1763
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9(6):653–660
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3(6):401–410
Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada KM (2005) A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol 170(5):793–802
Petrie RJ, Doyle AD, Yamada KM (2009) Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol 10(8):538–549
Chung CY, Lee S, Briscoe C, Ellsworth C, Firtel RA (2000) Role of Rac in controlling the actin cytoskeleton and chemotaxis in motile cells. Proc Natl Acad Sci USA 97(10):5225–5230
Sasaki AT, Chun C, Takeda K, Firtel RA (2004) Localized Ras signaling at the leading edge regulates PI3 K, cell polarity, and directional cell movement. J Cell Biol 167(3):505–518
Funamoto S, Meili R, Lee S, Parry L, Firtel RA (2002) Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109(5):611–623
Fink RD, Trinkaus JP (1988) Fundulus deep cells: directional migration in response to epithelial wounding. Dev Biol 129(1):179–190
Chirco R, Liu XW, Jung KK, Kim HR (2006) Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 25(1):99–113
Bertaux B, Hornebeck W, Eisen AZ, Dubertret L (1991) Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J Invest Dermatol 97(4):679–685
Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A (1994) Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2). J Cell Sci 107(Pt 9):2373–2379
Nagase H (1998) Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res 8(3):179–186
Ma DH, Chen JI, Zhang F, Hwang DG, Chen JK (2003) Inhibition of fibroblast-induced angiogenic phenotype of cultured endothelial cells by the overexpression of tissue inhibitor of metalloproteinase (TIMP)-3. J Biomed Sci 10(5):526–534
Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC (1999) Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer 79(9–10):1347–1355
Ahonen M, Baker AH, Kahari VM (1998) Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res 58(11):2310–2315
Baker AH, Zaltsman AB, George SJ, Newby AC (1998) Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest 101(6):1478–1487
Spurbeck WW, Ng CY, Strom TS, Vanin EF, Davidoff AM (2002) Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood 100(9):3361–3368
Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR (1994) Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem 269(12):9352–9360
Apte SS, Olsen BR, Murphy G (1995) The gene structure of tissue inhibitor of metalloproteinases (TIMP)-3 and its inhibitory activities define the distinct TIMP gene family. J Biol Chem 270(24):14313–14318
Anand-Apte B, Bao L, Smith R, Iwata K, Olsen BR, Zetter B, Apte SS (1996) A review of tissue inhibitor of metalloproteinases-3 (TIMP-3) and experimental analysis of its effect on primary tumor growth. Biochem Cell Biol 74(6):853–862
Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B (2003) A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med 9(4):407–415
Anand-Apte B, Pepper MS, Voest E, Montesano R, Olsen B, Murphy G, Apte SS, Zetter B (1997) Inhibition of angiogenesis by tissue inhibitor of metalloproteinase-3. Invest Ophthalmol Vis Sci 38(5):817–823
Van Muijen GN, Cornelissen LM, Jansen CF, Figdor CG, Johnson JP, Brocker EB, Ruiter DJ (1991) Antigen expression of metastasizing and non-metastasizing human melanoma cells xenografted into nude mice. Clin Exp Metastasis 9(3):259–272
Westphal JR, van’t Hullenaar RG, van der Laak JA, Cornelissen IM, Schalkwijk LJ, van Muijen GN, Wesseling P, de Wilde PC, Ruiter DJ, de Waal RM (1997) Vascular density in melanoma xenografts correlates with vascular permeability factor expression but not with metastatic potential. Br J Cancer 76(5):561–570
van Horssen R, Galjart N, Rens JA, Eggermont AM, ten Hagen TL (2006) Differential effects of matrix and growth factors on endothelial and fibroblast motility: application of a modified cell migration assay. J Cell Biochem 99(6):1536–1552
Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52(11):2745–2756
Lambert E, Dasse E, Haye B, Petitfrere E (2004) TIMPs as multifacial proteins. Crit Rev Oncol Hematol 49(3):187–198
Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, Baylin SB, Graff JR (1999) Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res 59(4):798–802
Wild A, Ramaswamy A, Langer P, Celik I, Fendrich V, Chaloupka B, Simon B, Bartsch DK (2003) Frequent methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene in pancreatic endocrine tumors. J Clin Endocrinol Metab 88(3):1367–1373
Anania MC, Sensi M, Radaelli E, Miranda C, Vizioli MG, Pagliardini S, Favini E, Cleris L, Supino R, Formelli F, Borrello MG, Pierotti MA, Greco A (2011) TIMP3 regulates migration, invasion and in vivo tumorigenicity of thyroid tumor cells. Oncogene 30(27):3011–3023
Liu S, Ren S, Howell P, Fodstad O, Riker AI (2008) Identification of novel epigenetically modified genes in human melanoma via promoter methylation gene profiling. Pigment Cell Melanoma Res 21(5):545–558
Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M (2000) Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer 85(2):182–188
Varney ML, Johansson SL, Singh RK (2005) Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma: role of monocyte chemotactic protein-1 and vascular endothelial growth factor-A. Melanoma Res 15(5):417–425
Streit M, Detmar M (2003) Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene 22(20):3172–3179
van der Laak JA, Westphal JR, Schalkwijk LJ, Pahlplatz MM, Ruiter DJ, de Waal RM, de Wilde PC (1998) An improved procedure to quantify tumour vascularity using true colour image analysis. Comparison with the manual hot-spot procedure in a human melanoma xenograft model. J Pathol 184(2):136–143
Hofmann UB, Westphal JR, Waas ET, Zendman AJ, Cornelissen IM, Ruiter DJ, van Muijen GN (1999) Matrix metalloproteinases in human melanoma cell lines and xenografts: increased expression of activated matrix metalloproteinase-2 (MMP-2) correlates with melanoma progression. Br J Cancer 81(5):774–782
Fassina G, Ferrari N, Brigati C, Benelli R, Santi L, Noonan DM, Albini A (2000) Tissue inhibitors of metalloproteases: regulation and biological activities. Clin Exp Metastasis 18(2):111–120
De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M, Van Riet I (2007) Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 92(4):440–449
Gialeli C, Theocharis AD, Karamanos NK (2011) Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278(1):16–27
Qi JH, Ebrahem Q, Ali M, Cutler A, Bell B, Prayson N, Sears J, Knauper V, Murphy G, Anand-Apte B (2013) Tissue inhibitor of metalloproteinases-3 peptides inhibit angiogenesis and choroidal neovascularization in mice. PLoS ONE 8(3):e55667
Stetler-Stevenson WG (2008) Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 1 (27): re6
Fernandez CA, Butterfield C, Jackson G, Moses MA (2003) Structural and functional uncoupling of the enzymatic and angiogenic inhibitory activities of tissue inhibitor of metalloproteinase-2 (TIMP-2): loop 6 is a novel angiogenesis inhibitor. J Biol Chem 278(42):40989–40995
Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG (2003) TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 114(2):171–180
Roland CL, Dineen SP, Lynn KD, Sullivan LA, Dellinger MT, Sadegh L, Sullivan JP, Shames DS, Brekken RA (2009) Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol Cancer Ther 8(7):1761–1771
Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA (2008) Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 68(11):4340–4346
Acknowledgments
We thank the Erasmus Medical Instrumentation Service (EMI) for assistance with the development of the migration barrier, and Michael van der Reijden from the Department of Cell Biology for technical assistance. This study was supported in part by the Stichting Erasmus Heelkundig Kankeronderzoek (SEHK) and EU FP6 ChemoRes LSHC-CT-2007-037665.
Conflict of interest
All authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 876 kb)
Supplementary material 2 (MOV 1019 kb)
Supplementary material 3 (MOV 465 kb)
Supplementary material 4 (MOV 1193 kb)
Supplementary material 5 (MOV 935 kb)
Supplementary material 6 (MOV 1001 kb)
Supplementary material 7 (MOV 695 kb)
Rights and permissions
About this article
Cite this article
Das, A.M., Seynhaeve, A.L.B., Rens, J.A.P. et al. Differential TIMP3 expression affects tumor progression and angiogenesis in melanomas through regulation of directionally persistent endothelial cell migration. Angiogenesis 17, 163–177 (2014). https://doi.org/10.1007/s10456-013-9385-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-013-9385-2