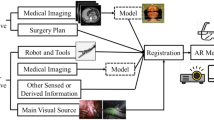
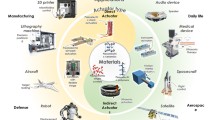
Abstract
Magnetic resonance imaging (MRI) is one of the most prevailing technologies to enable noninvasive and radiation-free soft tissue imaging. Operating a robotic device under MRI guidance is an active research area that has the potential to provide efficient and precise surgical therapies. MR-conditional actuators that can safely drive these robotic devices without causing safety hazards or adversely affecting the image quality are crucial for the development of MR-guided robotic devices. This paper aims to summarize recent advances in actuation methods for MR-guided robots and each MR-conditional actuator was reviewed based on its working principles, construction materials, the noteworthy features, and corresponding robotic application systems, if any. Primary characteristics, such as torque, force, accuracy, and signal-to-noise ratio (SNR) variation due to the variance of the actuator, are also covered. This paper concludes with a perspective on the current development and future of MR-conditional actuators.
























Similar content being viewed by others
References
Abdelaziz, M. E., V. Groenhuis, J. Veltman, F. Siepel, and S. Stramigioli. Controlling the Stormram 2: an MRI-compatible robotic system for breast biopsy. In 2017 IEEE International Conference on Robotics and Automation (ICRA). IEEE, pp. 1746–1753, 2017.
Alvara, A. N., T. Looi, R. Saab, A. Shorter, A. Goldenberg, and J. Drake. Development and validation of MRI compatible pediatric surgical robot with modular tooling for bone biopsy. In 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). IEEE, pp. 4935–4941, 2018.
ASTM International. ASTM F2503-13 Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment. West Conshohocken, PA: ASTM, 2013.
Bosboom, D. G. H., J. J. Fütterer, and J. Bosboom. D. G. H. Bosboom, J. J. Fütterer, and J. Bosboom, “Motor system, motor, and robot arm device comprising the same. Google Patents, 2016.
Bruyas, A., F. Geiskopf, L. Meylheuc, and P. Renaud. Combining multi-material rapid prototyping and pseudo-rigid body modeling for a new compliant mechanism. In 2014 IEEE International Conference on Robotics and Automation (ICRA). IEEE, pp. 3390–3396, 2014.
Bruyas, A., F. Geiskopf, and P. Renaud. Design and modeling of a large amplitude compliant revolute joint: the helical shape compliant joint. J. Mech. Des. 137(8):085003, 2015.
Burkhard, N., et al. A rolling-diaphragm hydrostatic transmission for remote MR-guided needle insertion. In 2017 IEEE International Conference on Robotics and Automation (ICRA). IEEE, pp. 1148–1153, 2017.
Chen, Y., I. S. Godage, S. Sengupta, C. L. Liu, K. D. Weaver, and E. J. Barth. MR-conditional steerable needle robot for intracerebral hemorrhage removal. Int. J. Comput. Assist. Radiol. Surg. 14(1):105–115, 2019.
Chen, Y., I. Godage, H. Su, A. Song, and H. Yu. Stereotactic systems for MRI-guided neurosurgeries: a state-of-the-art review. Ann. Biomed. Eng. 47(2):335–353, 2019.
Chen, Y., K.-W. Kwok, and Z. T. H. Tse. An MR-conditional high-torque pneumatic stepper motor for MRI-guided and robot-assisted intervention. Ann. Biomed. Eng. 42:1823–1833, 2014.
Chen, Y., C. D. Mershon, and Z. T. H. Tse. A 10-mm MR-conditional unidirectional pneumatic stepper motor. IEEE/ASME Trans. Mechatron. 20:782–788, 2015.
Chen, Y., et al. Robotic system for MRI-guided focal laser ablation in the prostate. IEEE/ASME Trans. Mechatron. 22:107–114, 2017.
Chen, Y., et al. MRI-guided robotically assisted focal laser ablation of the prostate using canine cadavers. IEEE Trans. Biomed. Eng. 65(7):1434–1442, 2017.
Cheng, S. S., Y. Kim, and J. P. Desai. New actuation mechanism for actively cooled SMA springs in a neurosurgical robot. IEEE Trans. Rob. 33(4):986–993, 2017.
Chinzei, K., and K. Miller. Towards MRI guided surgical manipulator. Med. Sci. Monit. 7:153–163, 2001.
Clark, J. E., et al. Biomimetic design and fabrication of a hexapedal running robot. In Proceedings 2001 ICRA. IEEE International Conference on Robotics and Automation (Cat. No. 01CH37164), vol. 4. IEEE, pp. 3643–3649, 2001.
Cole, G., H. S. K. Harrington, J. P. A. Camilo, and G. Fischer. Closedloop actuated surgical system utilizing real-time in-situ mri guidance. In ISER2010, 2010.
Comber, D. B., J. E. Slightam, V. R. Gervasi, J. S. Neimat, and E. J. Barth. Design, additive manufacture, and control of a pneumatic MR-compatible needle driver. IEEE Trans. Rob. 32(1):138–149, 2016.
Dong, Z., et al. High-performance continuous hydraulic motor for MR safe robotic teleoperation. IEEE Robot. Autom. Lett. 4(2):1964–1971, 2019.
Elbannan, K. M., and S. P. Salisbury. Design of a two degree-of-freedom, MRI-compatible actuator. In 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, pp. 940–943, 2012.
Elhawary, H., et al. A modular approach to mri-compatible robotics. EMBM 8:35–42, 2008.
Erwin, A., M. K. O’Malley, D. Ress, and F. Sergi. Kinesthetic feedback during 2DOF wrist movements via a novel MR-compatible robot. IEEE Trans. Neural Syst. Rehabil. Eng. 25(9):1489–1499, 2016.
Farimani, F. S., and S. Misra. Introducing PneuAct: parametrically-designed MRI-compatible pneumatic stepper actuator. In 2018 IEEE International Conference on Robotics and Automation (ICRA). IEEE, pp. 200–205, 2018.
Farrens, A. J., et al. Quantitative testing of fMRI-compatibility of an electrically active mechatronic device for robot-assisted sensorimotor protocols. IEEE Trans. Biomed. Eng. 2017. https://doi.org/10.1109/TBME.2017.2741346.
Fischer, G. S., G. Cole, and H. Su. Approaches to creating and controlling motion in MRI. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBC. IEEE, pp. 6687–6690, 2011.
Fischer, G. S., I. Iordachita, C. Csoma, J. Tokuda, S. P. DiMaio, C. M. Tempany, N. Hata, and G. Fichtinger. MRI-compatible pneumatic robot for transperineal prostate needle placement. IEEE/ASME Trans. Mechatron. 13(3):295–305, 2008.
Fischer, G. S., A. Krieger, I. Iordachita, C. Csoma, L. L. Whitcomb, and G. Fichtinger. MRI compatibility of robot actuation techniques—a comparative study. In International Conference on Medical Image Computing and Computer-Assisted Intervention, pp. 509–517, 2018.
Franco, E., D. Brujic, M. Rea, W. M. Gedroyc, and M. Ristic. Needle-guiding robot for laser ablation of liver tumors under MRI guidance. IEEE/ASME Trans. Mechatron. 21(2):931–944, 2015.
Fras, J., Y. Noh, H. Wurdemann, and K. Althoefer. Soft fluidic rotary actuator with improved actuation properties. In 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). IEEE, pp. 5610–5615, 2017.
Gassert, R., R. Moser, E. Burdet, and H. Bleuler. MRI/fMRI-compatible robotic system with force feedback for interaction with human motion. IEEE/ASME Trans. Mechatron. 11:216–224, 2006.
Gassert, R., A. Yamamoto, D. Chapuis, L. Dovat, H. Bleuler, and E. Burdet. Actuation methods for applications in MR environments (in English). Concept Magn. Reson. B 29B(4):191–209, 2006. https://doi.org/10.1002/Cmr.B.20070.
Gassert, R., et al. A 2-DOF fMRI compatible haptic interface to investigate the neural control of arm movements. In Proceedings 2006 IEEE International Conference on Robotics and Automation, 2006 (ICRA 2006), pp. 3825–3831, 2006.
Grand View Research. Image-guided Therapy Systems Market Size, Share & Trends Analysis Report By End Use (ASCs, Clinics, Hospitals), By Product (MRI, PET), By Application (Neurosurgery, Urology), and Segment Forecasts, 2018–2025 (accessed).
Groenhuis, V., F. J. Siepel, and S. Stramigioli. Dual-speed MR safe pneumatic stepper motors. In Robotics: Science and Systems, 2018.
Groenhuis, V., F. J. Siepel, J. Veltman, and S. Stramigioli. Design and characterization of Stormram 4: an MRI-compatible robotic system for breast biopsy. In 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). IEEE, pp. 928–933, 2017.
Groenhuis, V., and S. Stramigioli. Laser-cutting pneumatics. IEEE/ASME Trans. Mechatron. 21:1604–1611, 2016.
Groenhuis, V., J. Veltman, F. J. Siepel, and S. Stramigioli. Stormram 3: a magnetic resonance imaging-compatible robotic system for breast biopsy. IEEE Robot. Autom. Mag. 24(2):34–41, 2017.
Guo, Z., T. Lun, Y. Chen, H. Su, D. Chan, and K. Kwok. Novel design of an MR-safe pneumatic stepper motor for MRI-guided robotic interventions. In Proceedings of The Hamlyn Symposium on Medical Robotics, 2016.
Guo, Z., et al. Compact design of a hydraulic driving robot for Intraoperative MRI-guided bilateral stereotactic neurosurgery. IEEE Robot. Autom. Lett. 3(3):2515–2522, 2018.
Hao, S., A. Camilo, G. A. Cole, H. Nobuhiko, C. M. Tempany, and G. S. Fischer. High-field MRI-compatible needle placement robot for prostate interventions. Stud. Health Technol. Inf. 163:623, 2011.
He, Z., et al. Design of a percutaneous MRI-guided needle robot with soft fluid-driven actuator. IEEE Robot. Autom. Lett., 2020. https://doi.org/10.1109/LRA.2020.2969929.
Ho, M., and J. P. Desai. Characterization of SMA actuator for applications in robotic neurosurgery. In 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE, pp. 6856–6859, 2009.
Ho, M., and J. P. Desai. Towards a MRI-compatible meso-scale SMA-actuated robot using PWM control. In 2010 3rd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics. IEEE, pp. 361–366, 2010.
Ho, M., A. B. McMillan, J. M. Simard, R. Gullapalli, and J. P. Desai. Toward a meso-scale SMA-actuated MRI-compatible neurosurgical robot. IEEE Trans. Rob. 28(1):213–222, 2011.
Hunter, I. W., J. M. Hollerbach, and J. Ballantyne. A comparative analysis of actuator technologies for robotics. Robot. Rev. 2:299–342, 1991.
Kim, Y., and J. P. Desai. Design and kinematic analysis of a neurosurgical spring-based continuum robot using SMA spring actuators. In 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). IEEE, pp. 1428–1433, 2015.
Kim, D., E. Kobayashi, T. Dohi, and I. Sakuma. A new, compact MR-compatible surgical manipulator for minimally invasive liver surgery. In International Conference on Medical Image Computing and Computer-Assisted Intervention, pp. 99–106, 2002.
Klinke, T., et al. Artifacts in magnetic resonance imaging and computed tomography caused by dental materials. PLoS ONE 7(2):e31766, 2012.
Krieger, A., et al. Development and preliminary evaluation of an actuated MRI-compatible robotic device for MRI-guided prostate intervention. In 2010 IEEE International Conference on Robotics and Automation (ICRA), pp. 1066–1073, 2010.
Krieger, A., et al. Development and evaluation of an actuated MRI-compatible robotic system for MRI-guided prostate intervention. IEEE/ASME Trans. Mechatron. 18(1):273–284, 2011.
Lathrop, R. A., D. C. Rucker, and R. J. Webster. Guidance of a steerable cannula robot in soft tissue using preoperative imaging and conoscopic surface contour sensing. In 2010 IEEE International Conference on Robotics and Automation. IEEE, pp. 5601–5606, 2010.
Lee, K., et al. MR safe robotic manipulator for MRI-guided intracardiac catheterization. IEEE/ASME Trans. Mechatron. 23(2):586–595, 2018. https://doi.org/10.1109/TMECH.2018.2801787.
Li, G., et al. Robotic system for MRI-guided stereotactic neurosurgery. IEEE Trans. Biomed. Eng. 62(4):1077–1088, 2014.
Masamune, K., et al. Development of an MRI-compatible needle insertion manipulator for stereotactic neurosurgery. J. Image Guided Surg. 1(4):242–248, 1995.
Maurin, B., et al. In vivo study of forces during needle insertions. In Perspective in Image-Guided Surgery, pp. 415–422, 2004.
Monfaredi, R., et al. A prototype body-mounted MRI-compatible robot for needle guidance in shoulder arthrography. In 5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics. IEEE, pp. 40–45, 2014.
Mueller, B. Additive manufacturing technologies—rapid prototyping to direct digital manufacturing. Assembly Automation 2012. https://doi.org/10.1108/aa.2012.03332baa.010.
Muntener, M., et al. Transperineal prostate intervention: robot for fully automated MR imaging—system description and proof of principle in a canine model. Radiology 247(2):543–549, 2008.
Nimsky, C., O. Ganslandt, B. von Keller, J. Romstöck, and R. Fahlbusch. Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology 233(1):67–78, 2004.
Nycz, C. J., et al. Mechanical validation of an MRI compatible stereotactic neurosurgery robot in preparation for pre-clinical trials. In 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS). IEEE, pp. 1677–1684, 2017, 2019.
Orgill, G., and J. F. Wilson. Finite deformations of nonlinear, orthotropic cylindrical shells. J. Appl. Mech. 53(2):257–265, 1986.
Pappafotis, N., W. Bejgerowski, R. Gullapalli, J. M. Simard, S. K. Gupta, and J. P. Desai. Towards design and fabrication of a miniature MRI-compatible robot for applications in neurosurgery. In ASME 2008 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. American Society of Mechanical Engineers Digital Collection, pp. 747–754, 2008.
Paynter, H. M. Low-cost pneumatic arthrobots powered by tug-and-twist polymer actuators. Proc. of Japan/USA Symposium on Flexible Automation, vol. 1, pp. 107–110, 1996.
Paynter, H. M. Thermodynamic treatment of tug-&-twist technology: Part 1. Thermodynamic tugger design. In Proc. of Japan/USA Symposium on Flexible Automation, vol. 1, pp. 111–117, 1996.
Peters, T., and K. Cleary. Image-Guided Interventions: Technology and Applications. New York: Springer, 2008.
Pfeil, M. S. A., F. Geiskopf, T. P. Pusch, L. Barbe, and P. Renaud. Hydraulically-actuated compliant revolute joint for medical robotic systems based on multimaterial additive manufacturing. In 2019 International Conference on Robotics and Automation, Palais des congres de Montreal, Montreal, Canada, 2019.
Pfeil, A., et al. A 3D-printed needle driver based on auxetic structure and inchworm kinematics. In ASME 2018 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. American Society of Mechanical Engineers Digital Collection, 2018.
Preston, R. C. Output Measurements for Medical Ultrasound. Berlin: Springer, 2012.
Pylatiuk, C., A. Kargov, I. Gaiser, T. Werner, S. Schulz, and G. Bretthauer. Design of a flexible fluidic actuation system for a hybrid elbow orthosis. In 2009 IEEE International Conference on Rehabilitation Robotics. IEEE, pp. 167–171, 2009.
Sajima, H., H. Kamiuchi, K. Kuwana, T. Dohi, and K. Masamune. MR-safe pneumatic rotation stepping actuator. J. Robot. Mechatron. 24:820–827, 2012.
Sajima, H., I. Sato, H. Yamashita, T. Dohi, and K. Masamune. Two-DoF non-metal manipulator with pneumatic stepping actuators for needle puncturing inside open-type MRI. In 2010 World Automation Congress, Kobe, pp. 1–6, 2012.
Secoli, R., M. Robinson, M. Brugnoli, and F. Rodriguez y Baena. A low-cost, high-fieldstrength magnetic resonance imaging–compatible actuator. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 229:215–224, 2015.
Seifabadi, R., et al. MRI robot for prostate focal laser ablation: an ex vivo study in human prostate. J. Imaging 4(12):140, 2018.
Senturk, Y. M., and V. Patoglu. Design and control of an MRI compatible series elastic actuator. In 2016 IEEE International Conference on Robotics and Biomimetics (ROBIO). IEEE, pp. 1473–1479, 2016.
Sergi, F., A. C. Erwin, and M. K. O’Malley. Interaction control capabilities of an MR-compatible compliant actuator for wrist sensorimotor protocols during fMRI. IEEE/ASME Trans. Mechatron. 20(6):2678–2690, 2015.
Stoianovici, D., A. Patriciu, D. Petrisor, D. Mazilu, and L. Kavoussi. A new type of motor: pneumatic step motor. IEEE/ASME Trans. Mechatron. 12:98–106, 2007.
Stoianovici, D., et al. MRI-safe robot for endorectal prostate biopsy. IEEE/ASME Trans. Mechatron. 19:1289–1299, 2014.
Stoianovici, D., et al. Multi-imager compatible, MR safe, remote center of motion needle-guide robot. IEEE Trans. Biomed. Eng. 65(1):165–177, 2017.
Su, H., J. Sandoval, M. Makhdoomi, G. Ferrigno, and E. De Momi. Safety-enhanced human-robot interaction control of redundant robot for teleoperated minimally invasive surgery. In 2018 IEEE International Conference on Robotics and Automation (ICRA). IEEE, 2018, pp. 6611–6616, 2018.
Su, H., J. Sandoval, P. Vieyres, G. Poisson, G. Ferrigno, and E. De Momi. Safety-enhanced collaborative framework for tele-operated minimally invasive surgery using a 7-DoF torque-controlled robot. Int. J. Control Autom. Syst. 16(6):2915–2923, 2018.
Su, H., et al. A MRI-guided concentric tube continuum robot with piezoelectric actuation: a feasibility study. In 2012 IEEE International Conference on Robotics and Automation. IEEE, pp. 1939–1945, 2012.
Su, H., et al. Piezoelectrically actuated robotic system for MRI-guided prostate percutaneous therapy. IEEE/ASME Trans. Mechatron. 20(4):1920–1932, 2014.
Sutherland, G. R., I. Latour, A. D. Greer, T. Fielding, G. Feil, and P. Newhook. An imageguided magnetic resonance-compatible surgical robot. Neurosurgery 62:286–293, 2008.
Tang, Z. J., S. Sugano, and H. Iwata. Design of an MRI compatible robot for finger rehabilitation. In 2012 IEEE International Conference on Mechatronics and Automation. IEEE, pp. 611–616, 2012.
Tokuda, J., et al. Preclinical evaluation of an MRI-compatible pneumatic robot for angulated needle placement in transperineal prostate interventions. Int. J. Comput. Assist. Radiol. Surg. 7(6):949–957, 2012.
Tsekos, N. V., A. Khanicheh, E. Christoforou, and C. Mavroidis. Magnetic resonance–compatible robotic and mechatronics systems for image-guided interventions and rehabilitation: a review study. Annu. Rev. Biomed. Eng. 9:351–387, 2007.
U.S. Food and Drug Administration. Establishing Safety and Compatibility of Passive Implants in the Magnetic Resonance (MR) Environment. Silver Spring: U.S. Food and Drug Administration, 2014.
Vartholomeos, P., L. Qin, and P. E. Dupont. MRI-powered actuators for robotic interventions. In 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems.. IEEE, pp. 4508–4515, 2011.
Vogan, J., et al. Manipulation in MRI devices using electrostrictive polymer actuators: With an application to reconfigurable imaging coils. In IEEE International Conference on Robotics and Automation, 2004. Proceedings. ICRA’04. 2004, vol. 3. IEEE, pp. 2498–2504, 2004.
Wang, Y., M. Shazeeb, C. Sotak, and G. Fischer. Optimization of piezoelectric motors to enhance mr compatibility for interventional devices. In ISMRM 2009, p. 4437, 2009.
Webster, III, R. J., J. M. Romano, and N. J. Cowan. Mechanics of precurved-tube continuum robots. IEEE Trans. Rob. 25(1):67–78, 2008.
Wei, Y., Y. Chen, Y. Yang, and Y. Li. Novel design and 3-D printing of nonassembly controllable pneumatic robots. IEEE/ASME Trans. Mechatron. 21(2):649–659, 2015.
Whitney, J. P., T. Chen, J. Mars, and J. K. Hodgins. A hybrid hydrostatic transmission and human-safe haptic telepresence robot. In 2016 IEEE International Conference on Robotics and Automation (ICRA). IEEE, pp. 690–695, 2016.
Wilson, J., and G. Orgill. Linear analysis of uniformly stressed, orthotropic cylindrical shells. J. Appl. Mech. 53(2):249–256, 1986.
Wineland, A., Y. Chen, B. Boland, K. Chan, and Z. Tse. Magnetic resonance conditional microinjector. J. Imaging 5(1):4, 2019.
Wineland, A., Y. Chen, and Z. Tse. Magnetic resonance imaging compatible pneumatic stepper motor with geneva drive. J. Med. Devices 10(2):020950, 2016.
Wood, R. J. The first takeoff of a biologically inspired at-scale robotic insect. IEEE Trans. Rob. 24(2):341–347, 2008.
Wu, D., et al. Remotely actuated needle driving device for MRI-guided percutaneous interventions. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society, pp. 1–7, 2019.
Yu, N., and R. Riener. N. Yu and R. Riener, Review on MR-compatible robotic systems. In The First IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics, 2006. BioRob 2006.. IEEE, pp. 661–665, 2006.
Acknowledgments
This work is funded by Arkansas Biosciences Institute grant and NIH R01EB025179 and R01EB020003 grants. The authors would like to thank Kashu Yamazaki and Brandon Robertson for assistance searching and screening papers for review.
Conflict of interest
None to declare.
Financial disclosure
None to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Daniel Elson oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, Q., Monfaredi, R., Musa, M. et al. MR-Conditional Actuations: A Review. Ann Biomed Eng 48, 2707–2733 (2020). https://doi.org/10.1007/s10439-020-02597-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02597-8