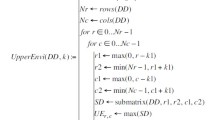Abstract
The objective of this study is to further investigate the ultrastructural details of the surface of Bowman’s membrane of the human cornea, using atomic force microscopy (AFM) images. One representative image acquired of Bowman’s membrane of a human cornea was investigated. The three-dimensional (3-D) surface of the sample was imaged using AFM in contact mode, while the sample was completely submerged in optisol solution. Height and deflection images were acquired at multiple scan lengths using the MFP-3D AFM system software (Asylum Research, Santa Barbara, CA), based in IGOR Pro (WaveMetrics, Lake Oswego, OR). A novel approach, based on computational algorithms for fractal analysis of surfaces applied for AFM data, was utilized to analyze the surface structure. The surfaces revealed a fractal structure at the nanometer scale. The fractal dimension, D, provided quantitative values that characterize the scale properties of surface geometry. Detailed characterization of the surface topography was obtained using statistical parameters, in accordance with ISO 25178-2: 2012. Results obtained by fractal analysis confirm the relationship between the value of the fractal dimension and the statistical surface roughness parameters. The surface structure of Bowman’s membrane of the human cornea is complex. The analyzed AFM images confirm a fractal nature of the surface, which is not taken into account by classical surface statistical parameters. Surface fractal dimension could be useful in ophthalmology to quantify corneal architectural changes associated with different disease states to further our understanding of disease evolution.







Similar content being viewed by others
References
Abrams, G. A., S. S. Schaus, S. L. Goodman, P. F. Nealey, and C. J. Murphy. Nanoscale topography of the corneal epithelial basement membrane and Descemet’s membrane of the human. Cornea 19(1):57–64, 2000.
Ali, S. H. R. Advanced nanomeasuring techniques for surface characterization. ISRN Optics 2012 859353:23, 2012. doi:10.5402/2012/859353.
Arffa, R. C. Grayson’s Diseases of the Cornea. St. Louis, MO: Mosby, 1997.
Atchison, D., and G. Smith. Optics of the Human Eye. Oxford, England: Butterworth Heineman, 2003.
Barnard, K., S. A. Burgess, D. A. Carter, and D. M. Woolley. Three-dimensional structure of type IV collagen in the mammalian lens capsule. J. Struct. Biol. 108(1):6–13, 1992.
Beuerman, R. W., and L. Pedroza. Ultrastructure of the human cornea. Microsc. Res. Tech. 33:320–335, 1996.
Chhabra, A., and R. V. Jensen. Direct determination of the f(alpha) singularity spectrum. Phys. Rev. Lett. 62:1327–1330, 1989.
Choi, S., H. J. Lee, Y. Cheong, J. H. Shin, K. H. Jin, H. K. Park, and Y. G. Park. AFM study for morphological characteristics and biomechanical properties of human cataract anterior lens capsules. Scanning 34(4):247–256, 2012. doi:10.1002/sca.21001.
Creasey, R., S. Sharma, J. E. Craig, C. T. Gibson, A. Ebner, P. Hinterdorfer, and N. H. Voelcker. Detecting protein aggregates on untreated human tissue samples by atomic force microscopy recognition imaging. Biophys. J. 99(5):1660–1667, 2010. doi:10.1016/j.bpj.2010.06.044.
Danysh, B. P., and M. K. Duncan. The lens capsule. Exp. Eye Res. 88(2):151–164, 2009.
Danysh, B., T. P. Patel, K. J. Czymmek, D. A. Edwards, L. Wang, J. Pande, and M. K. Duncan. Characterizing molecular diffusion in the lens capsule. Matrix Biol. 29(3):228–236, 2010. doi:10.1016/j.matbio.2009.12.004.
DelMonte, D., and T. Kim. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 37(3):588–598, 2011.
Doudevski, I., A. Rostagno, M. Cowman, J. Liebmann, R. Ritch, and J. Ghiso. Clusterin and complement activation in exfoliation glaucoma. Invest. Ophthalmol. Vis. Sci. 55(4):2491–2499, 2014. doi:10.1167/iovs.13-12941.
Giovanzana, S., H. T. Kasprzak, B. Pałucki, and Ş. Ţălu. Non-rotational aspherical models of the human optical system. J. Mod. Optic. 60(21):1898–1904, 2013.
GraphPad InStat software, version 3.20 (GraphPad, San Diego, CA). Available from: http://www.graphpad.com/instat/instat.htm (last accessed September 10th, 2014).
ISO 25178-2: 2012. Geometrical product specifications (GPS)—Surface texture: Areal—Part 2: Terms, definitions and surface texture parameters. http://www.iso.org (last accessed September 10th, 2014).
Komai, Y., and T. Ushiki. The three-dimesional organization of collagen fibrils in the human cornea and sclera. Invest. Ophth. Vis. Sci. 32(8):2244–2258, 1991.
Krachmer, J. H., R. S. Feder, and M. W. Belin. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 28(4):293–322, 1984.
Krachmer, J. H., M. J. Mannis, and E. J. Holland. Cornea (3rd ed.). St. Louis, MO: Mosby/Elsevier, 2011.
Leach, R. Characterisation of Areal Surface Texture. Berlin: Springer, pp. 36–37, 2013. doi:10.1007/978-3-642-36458-7.
Lombardo, M., Ş. Ţălu, M. Ţălu, S. Serrao, and P. Ducoli. Surface roughness of intraocular lenses with different dioptric powers assessed by atomic force microscopy. J. Cataract. Refract. Surg. 36(9):1573–1578, 2010. doi:10.1016/j.jcrs.2010.06.031.
Losa, G. A., D. Merlini, T. F. Nonnenmacher, and E. Weibel. Mathematics and bioscience in interaction. Fractals in Biology and Medicine, Vol. IV, Basel: Birkhäuser, 2005.
Lou, S., X. Jiang, and P. J. Scott. Application of the morphological alpha shape method to the extraction of topographical features from engineering surfaces. Measurement 46(2):1002–1008, 2013.
Mallick, S. B., S. Bhagwandin, and A. Ivanisevic. Characterization of collagen fibers in Bruch’s membrane using chemical force microscopy. Anal. Bioanal. Chem. 386(3):652–657, 2006.
Mallick, S. B., and A. Ivanisevic. Study of the morphological and adhesion properties of collagen fibers in the Bruch’s membrane. J. Phys. Chem. B. 109(41):19052–19055, 2005.
MountainsMap® 7 Software (Digital Surf, Besançon, France). Available from: http://www.digitalsurf.fr (last accessed September 10th, 2014).
Napolitano, A., S. Ungania, and V. Cannata. Fractal dimension estimation methods for biomedical images. In: MATLAB—A Fundamental Tool for Scientific Computing and Engineering Applications, Vol. 3, edited by V. Katsikis. Rijeka: Intech, 2012, pp. 161–178. doi:10.5772/48760.
Navarro, R. The optical design of the human eye: a critical review. J. Optom. 02(1):3–18, 2009.
Pandolfi, A., and G. A. Holzapfel. Three-dimensional modeling and computational analysis of the human cornea considering distributed collagen fibril orientations. J. Biomech. Eng. 130(6):061006, 2008.
Patel, S., D. Z. Reinstein, R. H. Silverman, and D. J. Coleman. The shape of Bowman’s layer in the human cornea. J. Refract. Surg. 14(6):636–640, 1998.
Perrier, E., A. Tarquis, and A. Dathe. A program for fractal and multifractal analysis of two-dimensional binary images: computer algorithms versus mathematical theory. Geoderma 134:284–294, 2006.
Roerdink, J. B. T. M., and A. Meijster. The watershed transform: definitions, algorithms, and parallelization strategies. Fundam. Inform. 41:187–228, 2000.
Sofou, A., and P. Maragos. Generalized flooding and Multicue PDE-based image segmentation. IEEE Trans. Image Process. 17(3):364–376, 2008.
Stach, S., S. Roskosz, J. Cybo, and J. Cwajna. Properties of sialon ceramics evaluated by means of multifractal, surface stereometry and quantitative fractography techniques. Mater. Charact. 60:1151–1157, 2009.
Ţălu, S. D. Ophtalmologie – Cours. Cluj-Napoca: Medical Publishing House “Iuliu Haţieganu”, 2005.
Ţălu, Ş. Mathematical methods used in monofractal and multifractal analysis for the processing of biological and medical data and images. Anim. Biol. Anim. Husb. 4(1):1–4, 2012.
Ţălu, Ş. Texture analysis methods for the characterisation of biological and medical images. Extreme Life Biospeol. Astrobiol. 4(1):8–12, 2012.
Ţălu, Ş. Multifractal geometry in analysis and processing of digital retinal photographs for early diagnosis of human diabetic macular edema. Curr. Eye Res. 38(7):781–792, 2013. doi:10.3109/02713683.2013.779722.
Ţălu, Ş., and S. Giovanzana. Fractal and multifractal analysis of human retinal vascular network: a review. Hum. Vet. Med. 3(3):205–212, 2011.
Ţălu, Ş. Characterization of retinal vessel networks in human retinal imagery using quantitative descriptors. Human & Veterinary Medicine – Bioflux 5(2):52-57, 2013.
Ţălu, Ş., and S. Stach. Multifractal characterization of unworn hydrogel contact lens surfaces. Polym. Eng. Sci. 54(5):1066–1080, 2014. doi:10.1002/pen.23650.
Ţălu, Ş., and M. Ţălu. An overview on mathematical models of human corneal surface. Int. Conf. Adv. Med. Health Care Technol. IFMBE Proc. 26:291–294, 2009.
Tao, A., J. Wang, Q. Chen, M. Shen, F. Lu, S. R. Dubovy, and M. A. Shousha. Topographic thickness of Bowman’s layer determined by ultra-high resolution spectral domain-optical coherence tomography. Invest. Ophth. Vis. Sci. 52(6):3901–3907, 2011.
Trattler, W. B., P. A. Majmudar, J. I. Luchs, and T. S. Swartz (eds.). Cornea Handbook. Thorofare, NJ: Slack Inc., 2010.
Wilson, S. E., and J. W. Hong. Bowman’s layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea 19:417–420, 2000.
Ziebarth, N. M., F. Rico, and V. Moy. Structural and mechanical mechanisms of ocular tissues probed by AFM. In: Applied Scanning Probe Methods, Vol. 14–16, edited by B. Bhushan. Heidelberg: Springer, 2010.
Acknowledgments
The donor human cornea was provided by Elizabeth Fout-Caraza of the Florida Lions Eye Bank. No funding was received from any of the following organizations: National Institutes of Health (NIH); Wellcome Trust; Howard Hughes Medical Institute (HHMI); and other(s).
Conflict of interest
Neither author has a financial or proprietary interest in any material or method mentioned. All authors read and approved the final manuscript. The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Estefanía Peña oversaw the review of this article.
Appendix
Appendix
The statistical parameters of 3-D surface roughness, according with ISO 25178-2:2012 are defined as follows:16
a) Height Parameters
Height parameters are a class of surface finish parameters that quantify the Z-axis perpendicular to the surface.
- (Sq):
-
Root mean square height is the standard deviation of the height distribution, or RMS surface roughness
- (Ssk):
-
Skewness is the third statistical moment, quantifying the symmetry of the height distribution. Negative skew indicates a predominance of valleys, while positive skew is seen on surfaces with peaks
- (Sku):
-
Kurtosis is the fourth statistical moment, quantifying the flatness of the height distribution. For spiky surfaces, Sku > 3; for bumpy surfaces, Sku < 3; perfectly random surfaces have kurtosis of 3
- (Sp):
-
Maximum peak height is the height between the highest peak and the mean plane
- (Sv):
-
Maximum pit height is the depth between the mean plane and the deepest valley
- (Sz):
-
Maximum height is the height between the highest peak and the deepest valley
- (Sa):
-
Arithmetical mean height is the mean surface roughness
b) Functional Parameters
Functional parameters are calculated from the Abbott–Firestone curve obtained by the integration of height distribution on the whole surface.
- (Smr):
-
Areal material ratio is the bearing area ratio at a given height. Ratio of the area of the material at a specified height c (cut level) to the evaluation area. The Smr(c) is expressed as a percentage. For the Smr parameter, the height c is counted by default from the mean plane. In our study, c = 1 μm under the highest peak
- (Smc):
-
Inverse areal material ratio is the height c at which a given areal material ratio p is satisfied. The height is calculated from the mean plane. In our study, p = 10%
- (Sxp):
-
Extreme peak height is the difference in height between q% and p% material ratio. This parameter must be configured with two thresholds entered in %. In our study, p = 50%, q = 97.5%
c) Spatial Parameters
Spatial parameters describe topographic characteristics based upon spectral analysis. They quantify the lateral information present on the X- and Y-axes of the surface.
- (Sal):
-
Auto-correlation length is the horizontal distance of the autocorrelation function (tx, ty) which has the fastest decay to a specified value s, with 0 < s < 1. The default value for s in the software is 0.2. This parameter expresses the content in wavelength of the surface. A high value indicates that the surface has mainly high wavelengths (low frequencies). In our study, s = 0.2
- (Str):
-
Texture-aspect ratio is the ratio of the shortest decrease length at 0.2 from the autocorrelation, on the greatest length. This parameter has a result between 0 and 1. If the value is near 1, we can say that the surface is isotropic, i.e., has the same characteristics in all directions. If the value is near 0, the surface is anisotropic, i.e., has an oriented and/or periodical structure. In our study, s = 0.2
- (Std):
-
Texture direction calculates the main angle for the texture of the surface, given by the maximum of the polar spectrum. This parameter has a meaning if Str is lower than 0.5. In our study, Reference Angle = 0°
d) Hybrid Parameters
Hybrid parameters are a class of surface finish parameters that quantify the information present on the X-, Y- and Z-axes of the surface, i.e., those criteria that depend both on the amplitude and the spacing, such as slopes, curvatures etc.
- (Sdq):
-
Root mean square gradient is the root-mean-square slope of the surface
- (Sdr):
-
Developed interfacial area ratio is the ratio of the increment of the interfacial area of the scale limited surface within the definition area over the definition area. The developed surface indicates the complexity of the surface thanks to the comparison of the curvilinear surface and the support surface. A completely flat surface will have a Sdr near 0%. A complex surface will have a Sdr of some percent value
e) Functional Volume Parameters
Functional volume parameters are typically used in tribological studies. They are calculated using the Abbott–Firestone curve (areal material ratio curve) calculated on the surface.
- Vm(p):
-
Material volume is the volume of the material at a material ratio p (in %). In our study, p = 10%
- Vv(p):
-
Void volume is the volume of the voids at a material ratio p (in %). In our study, p = 10%
- Vmp :
-
Peak material volume of the scale limited surface is the volume of material in the peaks, between 0% material ratio and a material ratio p (in %), calculated in the zone above c1. Vmp = Vm(p). In our study, p = 10%
- Vmc :
-
Core material volume of the scale limited surface is the volume of material in the core or kernel, between two material ratios p and q (in %), calculated in the zone between c1 and c2. Vmc = Vm(q) − Vm(p). In our study, p = 10%, q = 80%
- Vvc :
-
Core void volume of the scale limited surface is the volume of void in the core or kernel, between two material ratios p and q (in %), calculated in the zone between c1 and c2. Vvc = Vv(p) − Vv(q). In our study, p = 10%, q = 80%
- Vvv :
-
Pit void volume of the scale limited surface is the volume of void in the valleys, between a material ratio p (in %) and 100% material ratio, calculated in the zone below c2. Vvv = Vv(p). In our study, p = 80%
f) Feature Parameters
Feature parameters are derived from the segmentation of a surface into motifs (hills and dales). Segmentation is carried out in accordance with the watershed algorithm.
- Spd :
-
Density of peaks is the number of peaks per unit area. In our study, Spd (pruning = 5%)
- Spc :
-
Arithmetic mean peak curvature is the arithmetic mean of the principle curvatures of peaks within a definition area. In our study, Spc (pruning = 5%)
- S10z :
-
Ten point height is the average value of the heights of the five peaks with the largest global peak height added to the average value of the heights of the five pits with the largest global pit height, within the definition area. S10z = S5p + S5v. In our study, S10z (pruning = 5%)
- S5p :
-
Five point peak height is the average value of the heights of the five peaks with the largest global peak height, within the definition area. In our study, S5p (pruning = 5%)
- S5v :
-
Five point pit height is the average value of the heights of the five pits with the largest global pit height, within the definition area. In our study, S5v (pruning = 5%)
- Sda :
-
Closed dale area is the average area of dales connected to the edge at height c. In our study, Sda (pruning = 5%)
- Sha :
-
Closed hill area is the average area of hills connected to the edge at height c. In our study, Sha (pruning = 5%)
- Sdv :
-
Closed dale volume is the average volume of dales connected to the edge at height c. In our study, Sdv (pruning = 5%)
- Shv :
-
Closed hill volume is the average volume of hills connected to the edge at height c. In our study, Shv (pruning = 5%)
Rights and permissions
About this article
Cite this article
Ţălu, Ş., Stach, S., Sueiras, V. et al. Fractal Analysis of AFM Images of the Surface of Bowman’s Membrane of the Human Cornea. Ann Biomed Eng 43, 906–916 (2015). https://doi.org/10.1007/s10439-014-1140-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1140-3