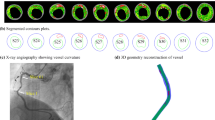
Abstract
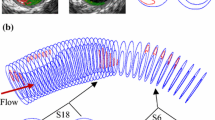
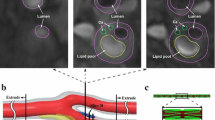
High mechanical stress in atherosclerotic plaques at vulnerable sites, called critical stress, contributes to plaque rupture. The site of minimum fibrous cap (FC) thickness (FCMIN) and plaque shoulder are well-documented vulnerable sites. The inherent weakness of the FC material at the thinnest point increases the stress, making it vulnerable, and it is the big curvature of the lumen contour over FC which may result in increased plaque stress. We aimed to assess critical stresses at FCMIN and the maximum lumen curvature over FC (LCMAX) and quantify the difference to see which vulnerable site had the highest critical stress and was, therefore, at highest risk of rupture. One hundred patients underwent high resolution carotid magnetic resonance (MR) imaging. We used 352 MR slices with delineated atherosclerotic components for the simulation study. Stresses at all the integral nodes along the lumen surface were calculated using the finite-element method. FCMIN and LCMAX were identified, and critical stresses at these sites were assessed and compared. Critical stress at FCMIN was significantly lower than that at LCMAX (median: 121.55 kPa; inter quartile range (IQR) = [60.70–180.32] kPa vs. 150.80 kPa; IQR = [91.39–235.75] kPa, p < 0.0001). If critical stress at FCMIN was only used, then the stress condition of 238 of 352 MR slices would be underestimated, while if the critical stress at LCMAX only was used, then 112 out of 352 would be underestimated. Stress analysis at FCMIN and LCMAX should be used for a refined mechanical risk assessment of atherosclerotic plaques, since material failure at either site may result in rupture.



Similar content being viewed by others
References
Aglyamov, S. R., A. R. Skovoroda, H. Xie, K. Kim, J. M. Rubin, M. O’Donnell, T. W. Wakefield, D. Myers, and S. Y. Emelianov. Model-based reconstructive elasticity imaging using ultrasound. Int. J. Biomed. Imaging 2007:11, 2007.
Bock, R. W., A. C. Gray-Weale, P. A. Mock, et al. The natural history of asymptomatic carotid artery disease. J. Vasc. Surg. 17:160–169, 1993 (discussion 170–171).
Boyle, J. J. Association of coronary plaque rupture and atherosclerotic inflammation. J. Pathol. 181:93–99, 1997.
Cai, J. M., T. S. Hatsukami, M. S. Ferguson, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 106:1368–1373, 2002.
Corti, R., R. Hutter, J. J. Badimon, and V. Fuster. Evolving concepts in the triad of atherosclerosis, inflammation and thrombosis. J. Thromb. Thrombolysis. 17:35–44, 2004.
de Putter, S., B. J. Wolters, M. C. Rutten, et al. Patient-specific initial wall stress in abdominal aortic aneurysms with a backward incremental method. J. Biomech. 40:1081–1090, 2007.
Falk, E., P. K. Shah, and V. Fuster. Coronary plaque disruption. Circulation 92:657–671, 1995.
Fung, Y. C. What are the residual stresses doing in our blood vessels? Ann. Biomed. Eng. 19:237–249, 1991.
Huang, X., C. Yang, C. Yuan, et al. Patient-specific artery shrinkage and 3D zero-stress state in multi-component 3D FSI models for carotid atherosclerotic plaques based on in vivo MRI data. Mol. Cell. Biomech. 6:121–134, 2009.
Lendon, C. L., M. J. Davies, G. V. Born, and P. D. Richardson. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis 87:87–90, 1991.
Lendon, C. L., M. J. Davies, P. D. Richardson, and G. V. Born. Testing of small connective tissue specimens for the determination of the mechanical behaviour of atherosclerotic plaques. J. Biomed. Eng. 15:27–33, 1993.
Li, Z. Y., S. Howarth, R. A. Trivedi, et al. Stress analysis of carotid plaque rupture based on in vivo high resolution MRI. J. Biomech. 39:2611–2622, 2006.
Li, Z. Y., T. Tang, J. U-King-Im, M. Graves, M. Sutcliffe, and J. H. Gillard. Assessment of carotid plaque vulnerability using structural and geometrical determinants. Circ. J. 72:1092–1099, 2008.
Redgrave, J. N., P. Gallagher, J. K. Lovett, and P. M. Rothwell. Critical cap thickness and rupture in symptomatic carotid plaques: the oxford plaque study. Stroke 39:1722–1729, 2008.
Richardson, P. D., M. J. Davies, and G. V. Born. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet 2:941–944, 1989.
Rothwell, P. M., M. Eliasziw, S. A. Gutnikov, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 361:107–116, 2003.
Speelman, L., E. M. Bosboom, G. W. Schurink, et al. Initial stress and nonlinear material behavior in patient-specific AAA wall stress analysis. J. Biomech. 42:1713–1719, 2009.
Takaya, N., C. Yuan, B. Chu, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI—initial results. Stroke 37:818–823, 2006.
Tang, T. Y., S. P. Howarth, Z. Y. Li, et al. Correlation of carotid atheromatous plaque inflammation with biomechanical stress: utility of USPIO enhanced MR imaging and finite element analysis. Atherosclerosis 196:879–887, 2008.
Tang, D., Z. Teng, G. Canton, et al. Sites of rupture in human atherosclerotic carotid plaques are associated with high structural stresses: an in vivo MRI-based 3D fluid–structure interaction study. Stroke 40:3258–3263, 2009.
Tang, D., Z. Teng, G. Canton, et al. Local critical stress correlates better than global maximum stress with plaque morphological features linked to atherosclerotic plaque vulnerability: an in vivo multi-patient study. Biomed. Eng. Online 8:15, 2009.
Tang, D., C. Yang, J. Zheng, et al. Local maximal stress hypothesis and computational plaque vulnerability index for atherosclerotic plaque assessment. Ann. Biomed. Eng. 33:1789–1801, 2005.
Trivedi, R. A., J. M. U-King-Im, M. J. Graves, et al. MRI-derived measurements of fibrous-cap and lipid-core thickness: the potential for identifying vulnerable carotid plaques in vivo. Neuroradiology 46:738–743, 2004.
Trivedi, R. A., J. M. U-King-Im, M. J. Graves, et al. Multi-sequence in vivo MRI can quantify fibrous cap and lipid core components in human carotid atherosclerotic plaques. Eur. J. Vasc. Endovasc. Surg. 28:207–213, 2004.
U-King-Im, J. M., T. Y. Tang, A. Patterson, et al. Characterisation of carotid atheroma in symptomatic and asymptomatic patients using high resolution MRI. J. Neurol. Neurosurg. Psychiatry 79:905–912, 2008.
van der Wal, A. C., A. E. Becker, C. M. van der Loos, and P. K. Das. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89:36–44, 1994.
Acknowledgments
This research is partly supported by ARTreat FP7 Europain Union and NIHR Cambridge Biomedical Research Centre. Dr. Umar Sadat is funded by the Medical Research Council & Royal College of Surgeons of England Joint Clinical Research Training Fellowship. We thank Tim Baynes from University of Cambridge for proof reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Teng, Z., Sadat, U., Li, Z. et al. Arterial Luminal Curvature and Fibrous-Cap Thickness Affect Critical Stress Conditions Within Atherosclerotic Plaque: An In Vivo MRI-Based 2D Finite-Element Study. Ann Biomed Eng 38, 3096–3101 (2010). https://doi.org/10.1007/s10439-010-0078-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-0078-3