Abstract
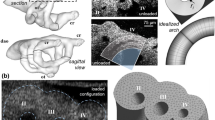
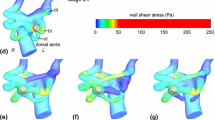
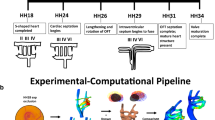
Morphogenesis of the “immature symmetric embryonic aortic arches” into the “mature and asymmetric aortic arches” involves a delicate sequence of cell and tissue migration, proliferation, and remodeling within an active biomechanical environment. Both patient-derived and experimental animal model data support a significant role for biomechanical forces during arch development. The objective of the present study is to quantify changes in geometry, blood flow, and shear stress patterns (WSS) during a period of normal arch morphogenesis. Composite three-dimensional (3D) models of the chick embryo aortic arches were generated at the Hamburger–Hamilton (HH) developmental stages HH18 and HH24 using fluorescent dye injection, micro-CT, Doppler velocity recordings, and pulsatile subject-specific computational fluid dynamics (CFD). India ink and fluorescent dyes were injected into the embryonic ventricle or atrium to visualize right or left aortic arch morphologies and flows. 3D morphology of the developing great vessels was obtained from polymeric casting followed by micro-CT scan. Inlet aortic arch flow and cerebral-to-lower body flow split was obtained from 20 MHz pulsed Doppler velocity measurements and literature data. Statistically significant variations of the individual arch diameters along the developmental timeline are reported and correlated with WSS calculations from CFD. CFD simulations quantified pulsatile blood flow distribution from the outflow tract through the aortic arches at stages HH18 and HH24. Flow perfusion to all three arch pairs are correlated with the in vivo observations of common pharyngeal arch defect progression. The complex spatial WSS and velocity distributions in the early embryonic aortic arches shifted between stages HH18 and HH24, consistent with increased flow velocities and altered anatomy. The highest values for WSS were noted at sites of narrowest arch diameters. Altered flow and WSS within individual arches could be simulated using altered distributions of inlet flow streams. Thus, inlet flow stream distributions, 3D aortic sac and aortic arch geometries, and local vascular biologic responses to spatial variations in WSS are all likely to be important in the regulation of arch morphogenesis.







Similar content being viewed by others
References
Butcher, J., D. Sedmera, R. E. Guldberg, R. R. Markwald, 2006, “Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography,” Developmental Dynamics, 236(3), pp. 802–809. doi:10.1002/dvdy.20962
Campbell, K. A., N. Hu, E. B. Clark, B. B. Keller, 1992, “Analysis of dynamic atrial dimension and function during early cardiac development in the chick embryo,” Pediatric Research, 32, pp. 333–337. doi:10.1203/00006450-199209000-00018
Dintenfass, L., 1985, Blood Viscosity, Hyperviscosity and Hyperviscosaemia, MTP Press (Kluwer), London.
Even-Tzur, N., Y. Kloog, M. Wolf, and D. Elad (2008) Mucus secretion and cytoskeletal modifications in cultured nasal epithelial cells exposed to wall shear stresses. Biophys J 95:2998–3008. doi:10.1529/biophysj.107.127142
Feintuch, A., P. Ruengsakulrach, A. Lin, J. Zhang, Y. Q. Zhou, J. Bishop, L. Davidson, D. Courtman, F. S. Foster, D. A. Steinman, R. M. Henkelman, C. R. Ethier 2007, “Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling,” Am. J. Physiol. Heart Circ. Physiol., 292, pp. H884-H892. doi:10.1152/ajpheart.00796.2006
Frakes, D. H., M. J. Smith, J. Parks, S. Sharma, S. M. Fogel, A. P. Yoganathan 2005, “New techniques for the reconstruction of complex vascular anatomies from MRI images,” J Cardiovasc Magn Reson, 7(2), pp. 425–432. doi:10.1081/JCMR-200053637
Gardiner, H., J. Brodszki, A. Eriksson, K. Marsál 2002, “Volume blood flow estimation in the normal and growth-restricted fetus,” Ultrasound Med Biol, 28(9), pp. 1107–1113. doi:10.1016/S0301-5629(02)00565-3
Greve, J. M., A. S. Les, B. T. Tang, M. T. D. Blomme, N. M. Wilson, R. L. Dalman, N. J. Pelc, C. A. Taylor, 2006, “Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics,” Am. J. Physiol. Heart Circ. Physiol., 291, pp. H1700-H1708. doi:10.1152/ajpheart.00274.2006
Groenendijk, B. C. W., B. P. Hierck, A. C. Gittenberger-de Groot, R. E. Poelmann, 2003, “Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos,” Dev. Dyn. 230, pp. 57–68. doi:10.1002/dvdy.20029
Groenendijk, B. C. W., S. Stekelenburg-de Vos, P. Vennemann, J. W. Wladimiroff, F. T. M. Nieuwstadt, R. Lindken, J. Westerweel, B. P. Hierck, N. T. C. Ursem, and R. E. Poelmann (2008) The endothelin-1 pathway and the development of cardiovascular defects in the haemodynamically challenged chicken embryo. J Vasc Res 45:54–68. doi:10.1159/000109077
Guldberg, R. E., C. L. Duvall, A. Peister, M. E. Oest, A. S. Lin, A. W. Palmer, M. E. Levenston 2008, “3D imaging of tissue integration with porous biomaterials,” Biomaterials, 29(28), pp. 3757–3761. doi:10.1016/j.biomaterials.2008.06.018
Hamburger, V., H. L. Hamilton 1951, “A series of normal stages in the development of the chick embryo,” Journal of Morphology, 88(1), pp. 49–92. doi:10.1002/jmor.1050880104
Himburg H. A., Dowd S. E., and Friedman M. H. (2007) Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol 293(1):H645–653. doi:10.1152/ajpheart.01087.2006
Hiruma, T., R. Hirakow, 1995, “Formation of the pharyngeal arch arteries in the chick embryo. Observations of corrosion casts by scanning electron microscopy,” Anat Embryol, 191, pp. 415–423. doi:10.1007/BF00304427
Hiruma, T., Y. Nakajima, H. Nakamura, 2002, “Development of pharyngeal arch arteries in early mouse embryo,” J. Anat., 201, pp. 15–29. doi:10.1046/j.1469-7580.2002.00071.x
Hogers, B., M. C. DeRuiter, A. M. J. Baasten, A. C. Gittenberger-de Groot, R. E. Poelmann 1995, “Intracardiac blood flow patterns related to the yolk sac circulation of the chick embryo,” Circ. Res., 76, pp. 871–877.
Hogers, B., M. C. DeRuiter, A. C. Gittenberger-de Groot and R. E. Poelmann (1997) Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ Res 80:473–481
Hogers, B., M. C. DeRuiter, A. C. Gittenberger-de Groot, R. E. Poelmann, 1999, “Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal,” Cardiovasc. Res., 41, pp. 87–99. doi:10.1016/S0008-6363(98)00218-1
Hove, J. R., R. W. Koster, A. S. Forouhar, G. Acevedo-Bolton, S. E. Fraser, M. Gharib, 2003, “Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis,” Nature, 421, pp. 172–177. doi:10.1038/nature01282
Hu, N., E. B. Clark, 1989, “Hemodynamics of the stage 12 to stage 29 chick embryo,” Circ Res., 65(6), pp. 1665–1670.
Hubbard, A. M., and Harty, P., 1999, “Prenatal magnetic resonance imaging of fetal anomalies,” Semin Roentgenol, 34(1), pp. 41–47. doi:10.1016/S0037-198X(99)80019-4
Huo, Y. X. G., G. S. Kassab, 2008, “The flow field along the entire length of mouse aorta and primary branches,” Ann. Biomed. Eng. 36, pp. 685–699. doi:10.1007/s10439-008-9473-4
Jin, S., J. Oshinski, D. P. Giddens, 2003, “Effects of wall motion and compliance on flow patterns in the ascending aorta,” J. Biomech. Eng. 125, pp. 347–354. doi:10.1115/1.1574332
Kurihara, Y., H. Kurihara, H. Oda, K. Maemura, R. Nagai, T. Ishikawa, Y. Yazaki, 1995, “Aortic arch malformation and ventricular septal defect in mice deficient in endothelin-1,” J. Clin. Invest., 96, pp. 293–300. doi:10.1172/JCI118033
Leuprecht, A., S. Kozerke, P. Boesiger, K. Perktold, 2003, “Blood flow in the human ascending aorta: a combined MRI and CFD study,” J. Eng. Math. 47, pp. 387–404. doi:10.1023/B:ENGI.0000007969.18105.b7
Leyhane J. C. (1969) Visualization of blood streams in the developing chick heart. Anat. Rec. 163:312–313.
McQuinn, T. C., M. Bratoeva, A. de Almeida, M. Remond, R. P. Thompson, D. Sedmera, 2007, “High-frequency ultrasonographic imaging of Avian Cardiovascular Development,” Dev. Dyn. 236, pp. 3503–3513. doi:10.1002/dvdy.21357
Morris, L., P. Delassus, A. Callanan, M. Walsh, F. Wallis, P. Grace, T. McGloughlin, 2005, “3-D numerical simulation of blood flow through models of the human aorta,” J. Biomech. Eng. 127, pp. 767–775. doi:10.1115/1.1992521
Mujumdar, R. B., L. A. Ernst, S. R. Mujumdar, C. J. Lewis, A. S. Waggoner, 1993, “Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters,” Bioconjug Chem., 4(2), pp. 105–111. doi:10.1021/bc00020a001
Ohno, M., Cooke, J. P., Dzau, V. J., and Gibbons, G. H., 1995, “Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade,” J Clin Invest, 95(3), pp. 1363-1369. doi:10.1172/JCI117787
Pekkan, K., D. de Zélicourt, L. Ge, F. Sotiropoulos, D. Frakes, M. A. Fogel, A. P. Yoganathan 2005, “Physics-driven CFD modeling of complex anatomical cardiovascular flows—a TCPC case study,” Ann Biomed Eng., 33(3), pp. 284–300. doi:10.1007/s10439-005-1731-0
Pekkan, K., L. P. Dasi, P. Nourparvar, S. Yerneni, K. Tobita, M. A. Fogel, B. B. Keller, A. Yoganathan, 2008, “In vitro hemodynamic investigation of the embryonic aortic arch at late gestation,” J. Biomech. 41, pp. 1697–1706. doi:10.1016/j.jbiomech.2008.03.013
Pekkan, K., O. Dur, K. Kanter, K. Sundareswaran, M. Fogel, A. Yoganathan, and A. Ündar (2008) Neonatal aortic arch hemodynamics and perfusion during cardiopulmonary bypass. J Biomech Eng 130:061012.
Peolma, C., P. Vennemann, R. Lindken, and J. Westerweel (2008) In vivo blood flow and wall shear stress measurements in the vitelline network. Exp Fluids 45:703–713
Poelmann, R. E., A. C. Gittenberger-de Groot, B. P. Hierck, 2008, “The development of the heart and microcirculation: role of shear stress,” Med Biol Eng Comput, 46, pp. 479–484. doi:10.1007/s11517-008-0304-4
Ravnic, D. J., Y. Z. Zhang, A. Tsuda, J. P. Pratt, H. T. Huss, S. J. Mentzer, 2006, “Multi-image particle tracking velocimetry of the microcirculation using fluorescent nanoparticles,” Microvasc. Res. 72, pp. 27–33. doi:10.1016/j.mvr.2006.04.006
Romanoff, A., 1961, Avian Embryo, Structural and Functional Development, Macmillan.
Rudolph, A. M., M. A. Heymann 1970, “Circulatory changes during growth in the fetal lamb,” Circ Res., 26(3), pp. 289–299.
Sadler T. W. (2005) Langman’s Medical Embryology. Lippincott Williams, Philadelphia, pp. 159–194
Schleich, J.-M., Pontchaillou, C. A., Dillenseger, J.-L., and Coatrieux, J.-L. (2002) Understanding normal cardiac development using animated models. IEEE Comput Graph Appl 22(1):14–19. doi:10.1109/38.974513
Sims, P. J., A. S. Waggoner, C.-H. Wang, J. F. Hoffman, 1974, “Studies on mechanism by which cyanine dyes measure membrane-potential in red blood-cells and phosphatidylcholine vesicles,” Biochemistry, 13(16), pp. 3315–3330. doi:10.1021/bi00713a022
Suo, J., D. E. Ferrara, E. Sorescu, R. E. Guldberg, W. R. Taylor, and D. P. Giddens (2007) Hemodynamic shear stress in mouse aortas implications for atherogenesis. Arterioscler Thromb Vasc Biol 27:346–351. doi:10.1161/01.ATV.0000253492.45717.46
Taber, L. A., N. Hu, T. Pexieder, E. B. Clark, B. B. Keller 1993, “Residual strain in the ventricle of the stage 16–24 chick embryo.,” Circ Res 72(2):455–462.
Ursell, P. C., J. M. Byrne, T. R. Fears, B. A. Strobino, W. M. Gersony 1991, “Growth of the great vessels in the normal human fetus and in the fetus with cardiac defects,” Circulation, 84(5), pp. 2028–2033.
Vennemann, P., K. T. Kiger, R. Lindken, B. C. W. Groenendijk, S. Stekelenburg-de Vos, T. L. M. ten Hagen, N. T. C. Ursem, R. E. Poelmann, J. Westerweel, B. P. Hierck, 2006, “In vivo micro particle image velocimetry measurements of blood-plasma in the embryonic avian heart,” J. Biomech. 39, pp. 1191–1200. doi:10.1016/j.jbiomech.2005.03.015
Waldo, K. L., M. R. Hutson, C.C. Ward, M. Zdanowicz, H. A. Stadt, D. Kumiski, R. Abu-Issa, M. L. Kirby 2005, “Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart,” Dev. Biol. 281, pp. 78–90. doi:10.1016/j.ydbio.2005.02.012
Wang, C., K. Pekkan, D. de Zelicourt, A. Parihar, A. Kulkarni, M. Horner, A. P. Yoganathan 2007, “Progress in the CFD Modeling of Flow Instability in Anatomical Total Cavopulmonary Connections,” Ann. Biomed. Eng. 35(11), pp. 1840–1856. doi:10.1007/s10439-007-9356-0
Yoshida, H., F. Manasek, R. A. Arcilla, 1983, “Intracardiac flow patterns in early embryonic life., “Circ. Res., 53, pp. 363–371
Yoshigi, M., Knott, G. D., Keller, B. B., 2000, “Lumped parameter estimation for the embryonic chick vascular system: a time-domain approach using MLAB,” Comput. Methods Programs Biomed. 63, pp. 29–41. doi:10.1016/S0169-2607(00)00061-4
20. Young, S., Kretlow, J. D., Nguyen, C., Bashoura, A. G., Baggett, L. S., Jansen, J. A., Wong, M., and Mikos, A. G. (2008) Microcomputed tomography characterization of neovascularization in bone tissue engineering application. Tissue Eng Part B Rev 14:295–306
Zamir, M., P. Sinclair, T. H. Wonnacott 1992, “Relation between diameter and flow in major branches of the arch of the aorta,” J Biomech, 25(11), pp. 1303–1310. doi:10.1016/0021-9290(92)90285-9
Ziegler T., Bouzourène K., Harrison V. J., Brunner H. R., and Hayoz D. (1998) Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 18(5):686–692
Acknowledgments
This research is supported by American Heart Association Beginning-Grant-in-Aid 0765284U (PI: Pekkan) and by the Children’s Hospital of Pittsburgh Foundation. Gratitude is expressed to Dr. Arvydas Usas for the micro-CT scanning. Dr. James Fitzpatrick, Dr. Greg Fisher, and Dr. Alan Waggoner provided valuable expertise on microscopy and fluorescent dye studies. We also acknowledge Pittsburgh Supercomputing Center Grant CCR080013 facilitating high-performance parallel CFD runs presented in this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (AVI 3260 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Dur, O., Patrick, M.J. et al. Aortic Arch Morphogenesis and Flow Modeling in the Chick Embryo. Ann Biomed Eng 37, 1069–1081 (2009). https://doi.org/10.1007/s10439-009-9682-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9682-5