Abstract
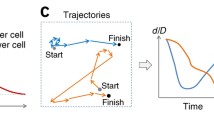
Cell migration paths of mammary epithelial cells (expressing different versions of the promigratory tyrosine kinase receptor Her2/Neu) were analyzed within a bimodal framework that is a generalization of the run-and-tumble description applicable to bacterial migration. The mammalian cell trajectories were segregated into two types of alternating modes, namely, the “directional mode” (mode I, the more persistent mode, analogous to the bacterial run phase) and the “re-orientation mode” (mode II, the less persistent mode, analogous to the bacterial tumble phase). Higher resolution (more pixel information, relative to cell size) and smaller sampling intervals (time between images) were found to give a better estimate of the deduced single cell dynamics (such as directional-mode time and turn angle distribution) of the various cell types from the bimodal analysis. The bimodal analysis tool permits the deduction of short-time dynamics of cell motion such as the turn angle distributions and turn frequencies during the course of cell migration compared to standard methods of cell migration analysis. We find that the 2-h mammalian cell tracking data do not fall into the diffusive regime implying that the often-used random motility expressions for mammalian cell motion (based on assuming diffusive motion) are invalid over the time steps (fraction of minute) typically used in modeling mammalian cell migration.












Similar content being viewed by others
References
Anderson A. R. A. 2005 A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Math. Med. Biol. 22, 163–186. 10.1093/imammb/dqi005
Anderson A. R. A., A. M. Weaver, P. T. Cummings, V. Quaranta 2006 Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 127, 905–915. 10.1016/j.cell.2006.09.042
Arrieumerlou C., T. Meyer 2005 A local coupling model and compass parameter for eukaryotic chemotaxis. Dev. Cell 8, 215–227. 10.1016/j.devcel.2004.12.007
Bargmann C. I., M. C. Hung, R. A. Weinberg 1986 Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell 45, 649–657. 10.1016/0092-8674(86)90779-8
Barton J. W., R. M. Ford 1995 Determination of effective transport coefficients for bacterial migration in sand columns. Appl. Environ. Microbiol. 61, 3329–3335
Berg H. C. 1975 Bacterial behavior. Nature 254, 389–392. 10.1038/254389a0
Berg H. C., D. A. Brown 1972 Chemotaxis in Escherichia coli analyzed by 3-dimensional tracking. Nature 239, 500–504. 10.1038/239500a0
Berg H. C., D. A. Brown 1974 Chemotaxis in Escherichia coli analyzed by three-dimensional tracking. Addendum. Antibiot. Chemother. 19, 55–78
Berg H. C., E. M. Purcell 1977 Physics of chemoreception. Biophys. J. 20, 193–219
Berg H. C., P. M. Tedesco 1975 Transient response to chemotactic stimuli in Escherichia coli. Proc. Natl. Acad. Sci. USA 72, 3235–3239. 10.1073/pnas.72.8.3235
Bergman A. J., K. Zygourakis 1999 Migration of lymphocytes on fibronectin-coated surfaces: temporal evolution of migratory parameters. Biomaterials 20, 2235–2244. 10.1016/S0142-9612(99)00154-4
Bray D. 2001 Cell Movements: From Molecules to Motility. Garland Publishing, New York, pp 372
Chambers A. F., A. C. Groom, I. C. MacDonald 2002 Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572. 10.1038/nrc865
Condeelis J., J. E. Segall 2003 Intravital imaging of cell movement in tumours. Nat. Rev. Cancer 3, 921–930. 10.1038/nrc1231
Dai L. S., W. Alt, K. Schilling, J. Retzlik, V. Gieselmann, T. M. Magin, J. Kappler 2005 A fast and robust quantitative time-lapse assay for cell migration. Exp. Cell Res. 311, 272–280. 10.1016/j.yexcr.2005.09.013
Debnath J., S. K. Muthuswamy, J. S. Brugge 2003 Morphogenesis and oncogenesis of mcf-10a mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268. 10.1016/S1046-2023(03)00032-X
Deisboeck T. S., T. Demuth, Y. Mansury 2005 Correlating velocity patterns with spatial dynamics in glioma cell migration. Acta Biotheor. 53, 181–190. 10.1007/s10441-005-2527-1
Dickinson R. B., R. T. Tranquillo 1993 Optimal estimation of cell-movement indexes from the statistical analysis of cell tracking data. AICHE J. 39, 1995–2010. 10.1002/aic.690391210
Dickinson R. B., R. T. Tranquillo 1995 Transport equations and indexes for random and biased cell migration based on single-cell properties. SIAM J. Appl. Math. 55, 1419–1454. 10.1137/S003613999223733X
Dimilla P. A., J. A. Quinn, S. M. Albelda, D. A. Lauffenburger 1992 Measurement of individual cell migration parameters for human tissue cells. AICHE J. 38, 1092–1104. 10.1002/aic.690380712
Duffy K. J., P. T. Cummings, R. M. Ford 1995 Random walk calculations for bacterial migration in porous media. Biophys. J. 68, 800–806
Duffy K. J., R. M. Ford 1997 Turn angle and run time distributions characterize swimming behavior for Pseudomonas putida. J. Bacteriol. 179, 1428–1430
Duffy K. J., R. M. Ford, P. T. Cummings 1997 Residence time calculation for chemotactic bacteria within porous media. Biophys. J. 73, 2930–2936
Dunn G. A. 1983 Characterising a kinesis response; time averaged measures of cell speed and directional persistence. Agents Actions Suppl. 12, 14–33
Dunn G. A., A. F. Brown 1987 A unified approach to analyzing cell motility. J. Cell Sci. 8, 81–102
Ford R. M., P. T. Cummings 1992 On the relationship between cell balance equations for chemotactic cell populations. SIAM J. Appl. Math. 52, 1426–1441. 10.1137/0152082
Ford, R. M., and P. T. Cummings. Mathematical models of bacterial chemotaxis. In: Chapman and Hall Microbiology Series; Mathematical Modeling in Microbial Ecology, edited by A. L. Koch, J. A. Robinson, and G. A. Milliken. New York: Chapman and Hall, 1998, pp. 228–269
Frymier P. D., R. M. Ford, H. C. Berg, P. T. Cummings 1995 3-dimensional tracking of motile bacteria near a solid planar surface. Proc. Natl. Acad. Sci. USA 92, 6195–6199. 10.1073/pnas.92.13.6195
Frymier P. D., R. M. Ford, P. T. Cummings 1993 Cellular dynamics simulations of bacterial chemotaxis. Chem. Eng. Sci. 48, 687–699. 10.1016/0009-2509(93)80136-E
Gail M. H., C. W. Boone 1970 Locomotion of mouse fibroblasts in tissue culture. Biophys. J. 10, 980–993
Harms B. D., G. M. Bassi, A. R. Horwitz, D. A. Lauffenburger 2005 Directional persistence of egf-induced cell migration is associated with stabilization of lamellipodial protrusions. Biophys. J. 88, 1479–1488. 10.1529/biophysj.104.047365
Ionides E. L., K. S. Fang, R. R. Isseroff, G. F. Oster 2004 Stochastic models for cell motion and taxis. J. Math. Biol. 48, 23–37. 10.1007/s00285-003-0220-z
Jourquin J., N. Yang, Y. Kam, C. Guess, V. Quaranta 2006 Dispersal of epithelial cancer cell colonies by lysophosphatidic acid (lpa). J. Cell. Physiol. 206, 337–346. 10.1002/jcp.20470
Kouvroukoglou S., K. C. Dee, R. Bizios, L. V. McIntire, K. Zygourakis 2000 Endothelial cell migration on surfaces modified with immobilized adhesive peptides. Biomaterials 21, 1725–1733. 10.1016/S0142-9612(99)00205-7
Kumar N., M. H. Zaman, H. D. Kim, D. A. Lauffenburger 2006 A high-throughput migration assay reveals her2-mediated cell migration arising from increased directional persistence. Biophys. J. 91, L32–L34. 10.1529/biophysj.106.088898
Le Douarin N. M. 1984 Cell migrations in embryos. Cell 38, 353–360. 10.1016/0092-8674(84)90490-2
Maheshwari G., H. S. Wiley, D. A. Lauffenburger 2001 Autocrine epidermal growth factor signaling stimulates directionally persistent mammary epithelial cell migration. J. Cell Biol. 155, 1123–1128. 10.1083/jcb.200109060
Martens L., G. Monsieur, C. Ampe, K. Gevaert, J. Vandekerckhove 2006 Cell_motility: a cross-platform, open source application for the study of cell motion paths. BMC Bioinformatics 7, 289–294. 10.1186/1471-2105-7-289
Othmer H. G., S. R. Dunbar, W. Alt 1988 Models of dispersal in biological systems. J. Math. Biol. 26, 263–298. 10.1007/BF00277392
Pankov R., Y. Endo, S. Even-Ram, M. Araki, K. Clark, E. Cukierman, K. Matsumoto, K. M. Yamada 2005 A rac switch regulates random versus directionally persistent cell migration. J. Cell Biol. 170, 793–802. 10.1083/jcb.200503152
Parkhurst M. R., W. M. Saltzman 1992 Quantification of human neutrophil motility in 3-dimensional collagen gels—effect of collagen concentration. Biophys. J. 61, 306–315
Quaranta V., A. M. Weaver, P. T. Cummings, A. R. A. Anderson 2005 Mathematical modeling of cancer: the future of prognosis and treatment. Clin. Chim. Acta 357, 173–179. 10.1016/j.cccn.2005.03.023
Rabut G., J. Ellenberg 2004 Automatic real-time three-dimensional cell tracking by fluorescence microscopy. J. Microsc. 216, 131–137
Rembold M., F. Loosli, R. J. Adams, J. Wittbrodt 2006 Individual cell migration serves as the driving force for optic vesicle evagination. Science 313, 1130–1134. 10.1126/science.1127144
Ridley A. J., M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons, A. R. Horwitz 2003 Cell migration: integrating signals from front to back. Science 302, 1704–1709. 10.1126/science.1092053
Rivero M. A., R. T. Tranquillo, H. M. Buettner, D. A. Lauffenburger 1989 Transport models for chemotactic cell populations based on individual cell behavior. Chem. Eng. Sci. 44, 2881–2897. 10.1016/0009-2509(89)85098-5
Sai J. Q., G. Walker, J. Wikswo, A. Richmond 2006 The il sequence in the llkil motif in cxcr2 is required for full ligand-induced activation of erk, akt, and chemotaxis in hl60 cells. J. Biol. Chem. 281, 35931–35941. 10.1074/jbc.M605883200
Schechter A. L., D. F. Stern, L. Vaidyanathan, S. J. Decker, J. A. Drebin, M. I. Greene, R. A. Weinberg 1984 The neu oncogene: an erb-b-related gene encoding a 185,000-mr tumor antigen. Nature 312, 513–516. 10.1038/312513a0
Schneider I. C., J. M. Haugh 2006 Mechanisms of gradient sensing and chemotaxis: conserved pathways, diverse regulation. Cell Cycle 5, 1130–1134
Selmeczi D., S. Mosler, P. H. Hagedorn, N. B. Larsen, H. Flyvbjerg 2005 Cell motility as persistent random motion: theories from experiments. Biophys. J. 89, 912–931. 10.1529/biophysj.105.061150
Stokes C. L., D. A. Lauffenburger, S. K. Williams 1991 Migration of individual microvessel endothelial cells: stochastic model and parameter measurement. J. Cell Sci. 99, 419–430
Ware M. F., A. Wells, D. A. Lauffenburger 1998 Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J. Cell Sci. 111, 2423–2432
Acknowledgments
This study was supported by NCI grant: Multiscale Mathematical Modeling of Cancer Invasion. Grant number: 5U54CA113007-02. We are grateful to Julie Maier and Brandy Weidow for technical assistance. The authors thank two anonymous reviewers whose thorough critiques of the original version of this manuscript led to significant improvements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
A key finding in bacterial chemotaxis is that the turn angle distribution is unaffected by the presence of a chemoattractant, while the run time distribution is modulated (bacteria extend their run times when moving in directions of increasing chemoattractant concentration),7 thus resulting in biased movement toward increasing chemoattractant concentration. Moreover, by demonstrating that the same run time increases were induced by a spatially homogeneous but time-varying chemoattractant concentration, Berg10 showed that E. coli were responding to the substantial derivative of the chemoattractant concentration (Dc/Dt, where c is the attractant concentration), so that the chemosensing mechanism in bacteria is related to the time rate of change in bound receptors on the cell surface. This rules out the possibility that in E. coli the chemosensory mechanism is based on the differences in the number of bound receptors over the cell surface (i.e., a direct sensing of the chemoattractant gradient by spatial comparison). A mathematical analysis of chemosensing by Berg and Purcell9 shows that despite the small cell size (∼1 μm) spatial sensing of a chemoattractant gradient is possible for E. coli; specifically, taking into account fluctuations in chemoattractant concentration on the spatial scale of a cell, Berg and Purcell derived expressions for the minimum time required for temporal sensing, T temporalsensing , and for spatial sensing, T spatialsensing , given by
where a is the radius of the cell, D is the self-diffusion coefficient of the chemoattractant, N is the number of receptors on the cell surface, s is the cell-receptor radius, \( \bar c \) is the equilibrium concentration of the chemoattractant, c 1/2 is the dissociation constant for the receptor-chemoattractant binding, and x is the direction in which the chemoattractant gradient exists. For temporal gradients created by the movement of the cell, \( (1/\bar c)(\partial \bar c/\partial t) = (v/\bar c)(\partial \bar c/\partial x), \) where v is the cell speed. For typical values of these parameters for E. coli responding to an aspartate gradient, Berg and Purcell found \( T_{{\text{sensing}}}^{{\text{temporal}}} > 0.4 - 1.4\;{\text{s}} \) (depending on magnitude of chemoattractant gradient) and for spatial sensing, \( T_{{\text{sensing}}}^{{\text{spatial}}} > 1.7\;{\text{s}}{\text{.}} \) Since the run lengths of flagellated bacteria are typically of the order of 1 s and longer, this analysis suggests that a bacterium could use either temporal or spatial sensing; however, because the swimming motion of a bacterium causes the cell body to rotate, the resulting disturbance to the surrounding liquid medium would create fluctuations in the chemoattractant gradient much larger than the gradient itself, thus ruling out the spatial sensing mechanism. To perform a similar analysis for eukaryotic cells, we use the experimental conditions of Sai et al.47 for the study of chemotaxis of HL60 cells stably expressing CXCR2 receptor in a microfluidic device-generated gradients of CXCL8 chemokine. For these cells in this chemotaxis assay, \( a = 7.5\,\mu {\text{m}},\;D = 10^{ - 6} \,{\text{cm}}^{\text{2}} {\text{/s}},\;\bar c = 1.25\,{\text{nM}},\;c_{1/2} = 1.5\,{\text{nM}},\;(1/\bar c)(\partial \bar c/\partial t) = (v/\bar c)(\partial \bar c/\partial x) = 2 \times 10^{ - 4} \,{\text{s}}^{ - 1} . \)Taking Ns/(Ns + πa) = 0.5 (a typical value), and using these values in Eqs. (1) and (2), we find that \( T_{{\text{sensing}}}^{{\text{temporal}}} > 1\,{\text{min}} \) and \( T_{{\text{sensing}}}^{{\text{spatial}}} > 18\,{\text{s}} .\) We note that the larger size of these cells (compared to bacteria) results in the time threshold for spatial sensing being less than that of temporal sensing; this is the reverse of the situation for bacteria, in which the time threshold for spatial sensing is greater than that of temporal sensing. From the bimodal analysis of the MCF-10A cells reported here, we find that the mean directional-mode time duration of these cancer cells ranges in several minutes compared to bacterial run times of seconds. Hence both temporal and spatial sensing mechanisms remain feasible for these eukaryotic cells.
Rights and permissions
About this article
Cite this article
Potdar, A.A., Lu, J., Jeon, J. et al. Bimodal Analysis of Mammary Epithelial Cell Migration in Two Dimensions. Ann Biomed Eng 37, 230–245 (2009). https://doi.org/10.1007/s10439-008-9592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9592-y