Abstract
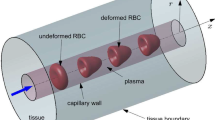
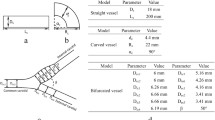
A detailed nonlinear four-region (red blood cell, plasma, interstitial fluid, and parenchymal cell) axially distributed convection-diffusion-permeation-reaction-binding computational model is developed to study the simultaneous transport and exchange of oxygen (O\(_{2}\)) and carbon dioxide (CO\(_{2}\)) in the blood–tissue exchange system of the heart. Since the pH variation in blood and tissue influences the transport and exchange of O\(_{2}\) and CO\(_{2}\) (Bohr and Haldane effects), and since most CO\(_{2}\) is transported as HCO\(_{3}^{-}\) (bicarbonate) via the CO\(_{2}\) hydration (buffering) reaction, the transport and exchange of HCO\(_{3}^{-}\) and H\(^{+}\) are also simulated along with that of O\(_{2}\) and CO\(_{2}\). Furthermore, the model accounts for the competitive nonlinear binding of O\(_{2}\) and CO\(_{2}\) with the hemoglobin inside the red blood cells (nonlinear \({\rm O_{2}\hbox{--}CO_{2}}\) interactions, Bohr and Haldane effects), and myoglobin-facilitated transport of O\(_{2}\) inside the parenchymal cells. The consumption of O\(_{2}\) through cytochrome-c oxidase reaction inside the parenchymal cells is based on Michaelis–Menten kinetics. The corresponding production of CO\(_{2}\) is determined by respiratory quotient (RQ), depending on the relative consumption of carbohydrate, protein, and fat. The model gives a physiologically realistic description of O\(_{2}\) transport and metabolism in the microcirculation of the heart. Furthermore, because model solutions for tracer transients and steady states can be computed highly efficiently, this model may be the preferred vehicle for routine data analysis where repetitive solutions and parameter optimization are required, as is the case in PET imaging for estimating myocardial O\(_{2}\) consumption.






Similar content being viewed by others
REFERENCES
Antonini, E., and M. Brunori. Hemoglobin and Myoglobin in their Reactions with Ligands. Amsterdam: North Holland, 1971, 436 pp.
Austin, W. H., E. Lacombe, P. W. Rand, and M. Chatterjee. Solubility of carbon dioxide in serum from 15 to 38\(^{\circ}\)C. J. Appl. Physiol. 18:301–304, 1963.
Barbee, J. H., and G. R. Cokelet. The Fahraeus effect. Microvasc. Res. 3:6–16, 1971.
Barta, E., S. Sideman, and J. B. Bassingthwaighte. Facilitated diffusion and membrane permeation of fatty acid in albumin solutions. Ann. Biomed. Eng. 28:331–345, 2000.
Bassingthwaighte, J. B. Plasma indicator dispersion in arteries of the human leg. Circ. Res. 19:332–346, 1966.
Bassingthwaighte, J. B., T. Yipintsoi, and R. B. Harvey. Microvasculature of the dog left ventricular myocardium. Microvasc. Res. 7:229–249, 1974.
Bassingthwaighte, J. B. A concurrent flow model for extraction during transcapillary passage. Circ. Res. 35:483–503, 1974.
Bassingthwaighte, J. B., T. Yipintsoi, and T. J. Knopp. Diffusional arteriovenous shunting in the heart. Microvasc. Res. 28:233–253, 1984.
Bassingthwaighte, J. B., and C. A. Goresky. Modeling in the analysis of solute and water exchange in the microvasculature. In: Handbook of Physiology. Sect. 2, The Cardiovascular System. Vol IV, The Microcirculation, edited by E. M. Renkin and C. C. Michel. Bethesda, MD: Am. Physiol. Soc., 1984, pp. 549–626.
Bassingthwaighte, J. B., F. P. Chinard, C. Crone, C. A. Goresky, N. A. Lassen, R. S. Reneman, and K. L. Zierler. Terminology for mass transport and exchange. Am. J. Physiol. Heart Circ. Physiol. 250:H539–H545, 1986.
Bassingthwaighte, J. B., C. Y. Wang, and I. S. Chan. Blood–tissue exchange via transport and transformation by endothelial cells. Circ. Res. 65:997–1020, 1989.
Bassingthwaighte, J. B., R. B. King, and S. A. Roger. Fractal nature of regional myocardial blood flow heterogeneity. Circ. Res. 65:578–590, 1989.
Bassingthwaighte, J. B., I. S. Chan, and C. Y. Wang. Computationally efficient algorithms for capillary convection-permeation-diffusion models for blood–tissue exchange. Ann. Biomed Eng. 20:687–725, 1992.
Bassingthwaighte, J. B., D. A. Beard, and Z. Li. The mechanical and metabolic basis of myocardial blood flow heterogeneity. Basic Res. Cardiol. 96:582–594, 2001.
Bassingthwaighte, J. B. The modelling of a primitive “sustainable” conservative cell. Philos. Trans. R. Soc. Lond. A 359:1055–1072, 2001.
Beard, D. A., and J. B. Bassingthwaighte. Advection and diffusion of substances in biological tissues with complex vascular networks. Ann. Biomed. Eng. 28:253–268, 2000.
Beard, D. A., and J. B. Bassingthwaighte. Modeling advection and diffusion of oxygen in complex vascular networks. Ann. Biomed. Eng. 29:298–310, 2001.
Beard, D. A. Computational framework for generating transport models from databases of microvascular anatomy. Ann. Biomed. Eng. 29:837–843, 2001.
Berzins, M., and P. M. Dew. Algorithm 690: Chebyshev polynomial software for elliptic-parabolic systems of PDEs. ACM Trans. Math. Softw. 17:178–206, 1994.
Beyer, R. P., J. B. Bassingthwaighte, and A. J. Deussen. A computational model of oxygen transport from red blood cells to mitochondria. Comput. Methods. Prog. Biomed. 67:39–54,2002.
Blom, J. G., and P. A. Zegeling. Algorithm 731: A moving grid-interface for systems of one-dimensional time-dependent partial differential equations. ACM Trans. Math. Softw. 20:194–214, 1994.
Buerk, D. G., and E. W. Bridges. A simplified algorithm for computing the variation in oxyhemoglobin saturation with pH \(P_{{\rm CO}_{2}}\), T and DPG. Chem. Eng. Commun. 47:113–124, 1986.
Butterworth, E., and Z. Li. JSim, a Java-based simulation system for modeling analysis of experimental data. http://nsr.bioeng.washington.edu/, Seattle, Washington:2000.
Caldwell, J. H., G. V. Martin, G. M. Raymond, and J. B. Bassingthwaighte. Regional myocardial flow and capillary permeability–surface area products are nearly proportional. Am. J. Physiol. Heart Circ. Physiol. 267:H654–H666, 1994.
Colombo, M. F., D. C. Rau, and V. A. Parsegian. Protein solvation in allosteric regulation: A water effect on hemoglobin. Science 256:655–659, 1992.
Dash, R. K., and J. B. Bassingthwaighte. Blood HbO\(_{2}\) and HbCO\(_{2}\) dissociation curves at varied O\(_{2}\), CO\(_{2}\), pH, 2,3-DPG and temperature levels. Ann. Biomed. Eng. 32:1676–1693, 2004.
Deussen, A., and J. B. Bassingthwaighte. Modeling [\(^{15}{\rm O}\)]oxygen tracer data for estimating oxygen consumption. Am. J. Physiol. Heart Circ. Physiol. 270:H1115–H1130,1996.
Dorson, W. J., Jr., and M. Voorhees. Limiting models for the transfer of CO\(_{2}\) and O\(_{2}\) in membrane oxygenators. Trans. Am. Soc. Artif. Intern. Organs 20A:219–226, 1974.
Federspiel, W. J., and I. H. Sarelius. An examination of the contribution of red cell spacing to the uniformity of oxygen flux at the capillary wall. Microvasc. Res. 27:273–285, 1984.
Federspiel, W. J., and A. S. Popel. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc. Res. 32:164–189, 1986.
Goldman, D., and A. S. Popel. A computational study of the effect of capillary network anastomoses and tortuosity on oxygen transport. J. Theor. Biol. 206:181–194, 2000.
Gonzalez, F., and J. B. Bassingthwaighte. Heterogeneities in regional volumes of distribution and flows in the rabbit heart. Am. J. Physiol. Heart Circ. Physiol. 258:H1012–H1024, 1990.
Goresky, C. A., G. G. Bach, and B. E. Nadeau. Red cell carriage of label: Its limiting effect on the exchange of materials in the liver. Circ. Res. 36:328–351, 1975.
Groebe, K., and G. Thews. Effects of red cell spacing and red cell movement upon oxygen release under conditions of maximally working skeletal muscle. In: Oxygen Transport to Tissue XI, edited by K. Rakusan, G. P. Biro, T. K. Goldstick, and Z. Turek. New York: Plenum Press, 1989, pp. 175–185.
Hedley-Whyte, J., and M. B. Laver. O\(_{2}\) solubility in blood and temperature correction factors for PO\(_{2}\). J. Appl. Physiol. 19:901–906, 1964.
Hellums, J. D. The resistance to oxygen transport in the capillaries relative to that in the surrounding tissue. Microvasc. Res. 13:131–136, 1977.
Hellums, J. D., P. K. Nair, N. S. Huang, and N. Ohshima. Simulation of intraluminal gas transport processes in the microcirculation. Ann. Biomed. Eng. 24:1–24, 1996.
Hill, A. V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 40:iv–vii, 1910.
Hill, E. P., G. G. Power, and L. D. Longo. A mathematical model of carbon dioxide transfer in the placenta and its interaction with oxygen. Am. J. Physiol. Cell Physiol. 224:283–299, 1973.
Hill, E. P., G. G. Power, and L. D. Longo. Mathematical simulation of pulmonary O\(_{2}\) and CO\(_{2}\) exchange. Am. J. Physiol. Cell Physiol. 224:904–917, 1973.
Hill, E. P., G. G. Power, and L. D. Longo. Kinetics of O\(_{2}\) and CO\(_{2}\) exchange. In: Bioengineering Aspects of the Lung, edited by J. B. West. New York: Marcel Dekker, 1977, pp. 459–514.
Hlastala, M. P., and R. D. Woodson. Saturation dependency of the Bohr effect: Interactions among H\(^{+}\), CO\(_{2}\) and DPG. J. Appl. Physiol. 38:1126–1131, 1975.
House, S. D., and H. H. Lipowsky. Microvascular hematocrit and red cell flux in rat cremaster muscle. Am. J. Physiol. Heart Circ. Physiol. 252:H211–H222, 1987.
Huang, N. S., and J. D. Hellums. A theoretical model for gas transport and acid/base regulation by blood flowing in microvessels. Microvasc. Res. 48:364–388, 1994.
Kelman, G. R. Digital computer subroutine for the conversion of oxygen tension into saturation. J. Appl. Physiol. 21:1375–1376, 1966.
Kettunen, M. I., O. H. J. Gröhn, M. J. Silvennoinen, M. Penttonen, and R. A. Kauppinen. Quantitative assesment of balance between oxygen delivery and consumption in the rat brain after transient ischemia with T\(_2\)-BOLD magnetic resonance imaging. J. Cereb. Blood Flow Metab. 22:262–270, 2002.
King, R. B., J. B. Bassingthwaighte, J. R. S. Hales, and L. B. Rowell. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ. Res. 57:285–295, 1985.
King, R. B., G. M. Raymond, and J. B. Bassingthwaighte. Modeling blood flow heterogeneity. Ann. Biomed. Eng. 24:352–372,1996.
Korzeniewski, B., and J. A. Zoladz. A model of oxidative phosphorylation in mammalian skeletal muscle. Biophys. Chem. 92:17–34, 2001.
Krogh, A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J. Physiol. (Lond.) 52:409–415, 1919.
Li, Z., T. Yipintsoi, and J. B. Bassingthwaighte. Nonlinear model for capillary–tissue oxygen transport and metabolism. Ann. Biomed. Eng. 25:604–619, 1997.
Nair, P. K., D. G. Buerk, and W. J. Whalen. Cat carotid body oxygen metabolism and chemoreception described by a two-cytochrome model. Am. J. Physiol. Heart Circ. Physiol. 250:H202–H207, 1986.
Ogawa, S., R. S. Menon, D. W. Tank, S. G. Kim, H. Merkle, J. M. Ellermann, and K. Ugurbil. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys. J. 64:803–812, 1993.
Popel, A. S. Theory of oxygen transport to tissue. Crit. Rev. Biomed. Eng. 17:257–321, 1989.
Rose, C. P., and C. A. Goresky. Limitations of tracer oxygen uptake in the canine coronary circulation. Circ. Res. 56:57–71, 1985.
Safford, R. E., and J. B. Bassingthwaighte. Calcium diffusion in transient and steady states in muscle. Biophys. J. 20:113–136, 1977.
Safford, R. E., E. A. Bassingthwaighte, and J. B. Bassingthwaighte. Diffusion of water in cat ventricular myocardium. J. Gen. Physiol. 72:513–538, 1978.
Salathe, E. P., R. Fayad, and S. W. Schaffer. Mathematical analysis of carbon dioxide transfer by blood. Math. Biosci. 57:109–153, 1981.
Schenkman, K. A., D. R. Marble, D. H. Burns, and E. O. Feigl. Myoglobin oxygen dissociation by multiwavelength spectroscopy. J. Appl. Physiol. 82:86–92, 1997.
Schenkman, K. A., D. R. Marble, D. H. Burns, and E. O. Feigl. Optical spectroscopic method for in vivo measurement of cardiac myoglobin oxygen saturation. Appl. Spectrosc. 53:332–338, 1999.
Secomb, T. W., R. Hsu, N. B. Beamer, and B. M. Coull. Theoretical simulation of oxygen transport to brain by networks of microvessels: Effects of oxygen supply and demand on tissue hypoxia. Microcirculation 7:237–247, 2000.
Shishido, F., K. Uemura, A. Inugami, T. Ogawa, T. Yamaguchi, I. Kanno, M. Murakami, K. Tagawa, and N. Yasui. Remote effects in MCA territory ischemic infarction: A study of regional cerebral blood flow and oxygen metabolism using positron computed tomography and \(^{15}\)O labeled gases. Radiat. Med. 5:36–41, 1987.
Singh, M. P., M. Sharan, and A. Aminataei. Development of mathematical formulae for O\(_{2}\) and CO\(_{2}\) dissociation curves in the blood. IMA J. Math. Appl. Med. Biol. 6:25–46, 1989.
Suenson, M., D. R. Richmond, and J. B. Bassingthwaighte. Diffusion of sucrose, sodium, and water in ventricular myocardium. Am. J. Physiol. 227:1116–1123, 1974.
Vinnakota, K., and J. B. Bassingthwaighte. Myocardial density and composition: A basis for calculating intracellular metabolite concentrations. Am. J. Physiol. Heart Circ. Physiol. 286:H1742–H1749, 2004.
Winslow, R. M., M. Samaja, N. J. Winslow, L. Rossi-Bernardi, and R. I. Shrager. Simulation of continuous blood O\(_{2}\) equilibrium over physiological pH, DPG, and PCO\(_{2}\) range. J. Appl. Physiol.: Respir. Environ. Exercise Physiol. 54:524–529, 1983.
Ye, G. F., T. W. Moore, D. G. Buerk, and D. Jaron. A compartmental model for oxygen-carbon dioxide coupled transport in the microcirculation. Ann. Biomed. Eng. 22:464–479, 1994.
ACKNOWLEDGMENTS
This research was supported by the National Simulation Resource Facility for Circulatory Mass Transport and Exchange via the grants P41-RR1243 from NCRR/NIH and R01-EB001973 from NIBIB/NIH. RKD was partially supported by the Center for Modeling Integrated Metabolic Systems (MIMS) via grant P50-GM66309 from NIGMS/NIH. The authors are grateful to the reviewers for their useful comments and suggestions. The model code is available at http://nsr.bioeng.washington.edu/ and can be run there via an applet supported by NIH/NIBIB R01-EB001973.
Author information
Authors and Affiliations
Corresponding author
APPENDIX: ERRATA IN THE 2004 PUBLICATION OF THE HbO2 AND HbCo2 DISSOCIATION CURVES
APPENDIX: ERRATA IN THE 2004 PUBLICATION OF THE HbO2 AND HbCo2 DISSOCIATION CURVES
Dash and Bassingthwaighte (2004): The article by Dash and Bassingthwaighte (Ann Biomed Eng 32: 1676–1693, 2004) appeared in print without galley proofs having been sent to either of the authors for review and correction. Errors in print setting were such that the equations essential to reproducing the scientific work need to be reprinted in order to make the present paper understandable and the original work reproducible. This appendix corrects the errors in the 2004 printing by providing the original, correct equations and text.
Correction 1: On page 1678 under MATHEMATICAL FORMULATIONS, Beginning at the end of paragraph 1, are a series of corrections to the equations 2a–f, where an upper case K is to be used instead of an erroneous lower case k:
“The governing reactions are:
CO2 hydration reaction – \({\rm HCO}_3^ -\) buffering of CO2:
CO2 binding to HmNH2 chains – HmNHCOO- buffering of CO2:
CO2 binding to O2HmNH2 chains – O2HmNHCOO- buffering of CO2:
O2 binding to HmNH2 chains – one-step kinetics using the \(P_{{\rm O}_2 }\)-dependent values of the rates of association and dissociation to accounts for the cooperativity.
Ionization of HmNH2 chains – pH buffering:
Ionization of O2HmNH2 chains – pH buffering:
Correction 2: On page 1679, in the last sentence of this same section, and ending just above the section heading “Equilibrium Relations” the “\(k' _4\)” should be replaced by the upper case \(K' _4\) in two places:
“To account for this through our one-step kinetic approach in reaction (2d), we use the equilibrium “constant” \(K' _4 ( = kf' _4 /kb' _4 )\) a function of O2 partial pressure \(P_{{\rm O}_2 }\); \(K' _4\) also depends on the levels of pH, \(P_{{\rm CO}_2 }\), 2,3-DPG concentration, and temperature inside the RBCs4.”
Correction 3: All of Equations 3 and 4 require corrections to the upper versus lower case Ks and the kfs in the section on Equilibrium Relations:
where the equilibrium constants \(K' _1 ,K' _2 ,K' _3\) and \(K' _4\) are defined by
Correction 4: In Appendix A, Eq. A.2 should be corrected to read:
Correction 5: The last sentence in the paragraph below Eq. A.3 should correct R rbc to read:
“The value of R rbc is about 0.6913–15,31,38. Multiplying [CO2]bl by 22.256 converts the units of molar to ml of CO2 per ml of blood.”
Correction 6: The first sentence of the paragraph above Eq. A.4 should correct H2O:
“From the data used in Hill et al.13–15, [H2O] \(kf' _1 \approx 0.12\) s-1 and \(kb' _1 \approx 89\) s-1, so \(K' _1 = [{\rm H}_2 {\rm O}]\;kf' _1 /kb' _1 \approx 1.35\times 10^{ - 3}\).”
Correction 7: In Appendix B, Eqs. B.1 to B.3 should be corrected re \(K' _{{\rm HbO}_2 }\) and K fact and the latter part of the first paragraph corrected to read:
“
where \(K' _{{\rm HbO}_2 }\) (with units \(M^{ - (1 + n_0 )}\)) is given by Equations 13 and 14:
The P 50 is defined as before by Eq. (11) and the kinetic terms K ratio and K fact are defined by Eqs. (18) and (14b). The Hill exponent in the expression for \(S_{{\rm HbO}_2 }\) is now 1+n 0 and apparent Hill coefficient \(K' _{{\rm HbO}_2 }\) is now independent of [O2]. This makes \(S_{{\rm HbO}_2 }\) analytically invertible, when \(P_{{\rm CO}_2 }\), pH, [DPG] and T are known. The inverted equation for [O2] is given by
It is clear from Eq. (2) that \(K' _{{\rm HbO}_2 }\) can be determined completely using only the P 50=P 50([H+], [CO2], [DPG], T) data which is given by Eq. (11). This avoids the calculations of K ratio and K fact which involves the complex computations of the empirical indices n 1, n 2, n 3, and n 4 using Eqs. (20a) to (20d). This also simplifies the computation of \(S_{{\rm HbO}_2 }\) from [O2] and vice-versa significantly. However, in the computation of \(S_{{\rm HbCO}_2 }\) from [CO2] and vice-versa (not shown in this appendix), the calculations of K fact and K ratio are essential as the P 50 data for 50% HbCO2 saturation is not available in the literature.”
Rights and permissions
About this article
Cite this article
Dash, R.K., Bassingthwaighte, J.B. Simultaneous Blood–Tissue Exchange of Oxygen, Carbon Dioxide, Bicarbonate, and Hydrogen Ion. Ann Biomed Eng 34, 1129–1148 (2006). https://doi.org/10.1007/s10439-005-9066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-9066-4