Abstract
Background: Gastric cancer is the most frequent gastrointestinal cancer in Mexico. Only 33% of cases are resectable. Our aim was to determine the activity and toxicity of the cisplatin, etoposide, leucovorin, and 5-fluorouracil combination in initially unresectable tumors and to determine its ability to permit resection.
Methods: Sixty patients with unresectable gastric adenocarcinoma were treated with cisplatin 80 mg/m2, etoposide 80 mg/m2, leucovorin 25 mg/m2, and 5-fluorouracil 800 mg/m2 by central intravenous catheter for 4 consecutive days. Two courses of this combination were followed by surgical resection.
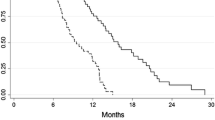
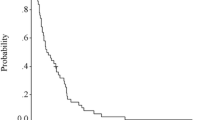
Results: The overall response rate was 36.8% (20 partial responses and one complete response). By using logistic regression analysis, the tumor, node, and metastasis stage (risk ratio, 2.04; 95% confidence interval, 1.03–4.02; P 5.039) was identified as the response determinant to chemotherapy. Major toxicity was grade 3 or 4 neutropenia in 67% of patients. Ten resections were performed (17.5%); five were curative and five palliative. Operative morbidity and mortality rates were 40% and 10%, respectively. The median length of survival was 7.46 and 13.3 months for nonresponders and responders, respectively (P 5.011).
Conclusions: The cisplatin, etoposide, leucovorin, and 5-fluorouracil combination is active in advanced gastric cancer and the toxicity level is acceptable. This treatment permits a 17.5% resection rate in previously unresectable tumors. A randomized trial of surgery vs. neoadjuvant chemotherapy plus surgery is warranted.
Similar content being viewed by others
REFERENCES
Secretaria de Salud. Estomago. In: Direccion General de Epidemiologia, Instituto Nacional de Cancerologia, and Asociacion Mexicana de Patologos, eds. Compendio del Registro Histopatologico de Neoplasias Malignas en Mexico. Mexico City, México: Secretaria de Salud, 1997:77–81.
Oñate-Ocaña LF, Mondragon-Sánchez R, Ruiz-Molina JM, Aiello-Crocifoglio V. Gastric cancer. Rev Gastroenterol Mex 1997;62:160–166.
Kelsen DP. Adjuvant and neoadjuvant chemotherapy for gastric cancer. Semin Oncol 1996;23:379–389.
Nakajima T, Ota K, Ishihara S, et al. Combined intensive chemotherapy and radical surgery for incurable gastric cancer. Ann Surg Oncol 1997;4:203–208.
Wilke H, Preusser P, Fink U, et al. New developments in the treatment of gastric carcinoma. Semin Oncol 1990;17:61–70.
American Joint Committee on Cancer. Stomach. In: Manual for Staging of Cancer. 4th ed. Philadelphia: JB Lippincott, 1992:63–67.
World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. WHO offset publication no. 48. Geneva: World Health Organization, 1979.
Japanese Research Society for Gastric Cancer. Japanese Classification of Gastric Carcinoma. 1st ed (in English). Tokyo: Kanehara, 1995.
Feinstein AR. Multiple logistic regression. In: Multivariable Analysis: An Introduction. New Haven, CT: Yale University Press, 1996:297–330.
Kaplan EL, Meier PM. Nonparametric estimation from incomplete observations. J Am Stat Soc 1958;53:457–481.
Hermans J, Bonenkampp JJ, Boon MC, et al. Adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of randomized trials. J Clin Oncol 1993;11:1441–1447.
Ajani JA, Mayer RJ, Ota DM, et al. Preoperative and postoperative combination chemotherapy for potentially resectable gastric carcinoma. J Natl Cancer Inst 1993;85:1839–1844.
Ajani JA, Ota DM, Jessup JM, et al. Resectable gastric carcinoma: an evaluation of preoperative and postoperative chemotherapy. Cancer 1991;68:1501–1506.
Leichman L, Silverman H, Leichman CG, et al. Preoperative systemic chemotherapy followed by adjuvant postoperative intraperitoneal therapy for gastric cancer: a University of Southern California pilot program. J Clin Oncol 1992;10:1933–1942.
Kelsen D, Karpeh M, Schwartz G, et al. Neoadjuvant and postoperative chemotherapy for high-risk gastric cancer (abstract). Proc Am Soc Clin Oncol 1994;13:195.
Nakajima T. Tabular analysis of 10000 cases of gastric cancer (in Japanese). Jpn J Cancer Chemother 1994;21:1813–1897.
Hansen RM. 5-Fluorouracil by protracted venous infusion: a review of recent clinical studies. Cancer Invest 1991;9:637–642.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallardo-Rincón, D., Oñate-Ocaña, L.F. & Calderillo-Ruiz, G. Neoadjuvant Chemotherapy With P-ELF (Cisplatin, Etoposide, Leucovorin, 5-Fluorouracil) Followed by Radical Resection in Patients With Initially Unresectable Gastric Adenocarcinoma: A Phase II Study. Ann Surg Oncol 7, 45–50 (2000). https://doi.org/10.1007/s10434-000-0045-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10434-000-0045-6