Abstract
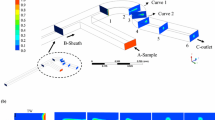
A microfluidic manifold has been designed, fabricated, and tested that hydrodynamically focuses a sample into the center of a microchannel and provides control over the vertical position of the sample via the flow-rates of the focusing fluids. To characterize the focusing action, a mixing experiment was performed in which the sample fluid and focusing fluid contained different fluorescent dyes. By sweeping the ratio of the rate of the top focusing fluid to the rate of the bottom focusing fluid, the sample was positioned first near the top of the microchannel and then translated downward in steps to the bottom of the microchannel. Images were obtained with confocal microscopy, and the presumptive concentration distributions were computed using multiphysics software. The simulations were shown by direct visual comparison with the experimental images to accurately predict the distributions of fluids in our device. In order to quantitatively compare the two data sets, the images and simulations were analyzed using a simple center-of-mass measurement, and according to this measurement, the simulations accurately predicted the vertical position the focused sample.






Similar content being viewed by others
Notes
A single-layer planar microfluidic device has also been used to focus a sample into the center of a microchannel (Mao et al. 2007); however, it remains unclear whether this device can be used to control the vertical position of the sample via straightforward adjustment to the relative flow-rates due to its reliance on the transverse transport mechanism popularly known as “microfluidic drifting”.
If desired, the total number of flow-cell inputs could be reduced from five to three by connecting, on-chip, the focusing inputs for each focusing stage (see Chang et al. 2007). In this case, the total flow-rate would be U 0 = U 1 + U 2 + U 3.
References
Chang C-C, Huang Z-X, Yang R-J (2007) Three-dimensional hydrodynamic focusing in two-layer polydimethylsiloxane microchannels. J Micromech Microeng 17(8):1479–1486
Dickinson ME et al (2001) Multi-spectral imaging and linear unmixing add a whole new dimension to laser scanning fluorescence microscopy. Biotechniques 31(6):1272–1279
Fu L-M, Yang R-J, Lin C-H, Pan Y-J, Lee G-B (2004) Electrokinetically driven micro flow cytometers with integrated fiber optics for on-line cell/particle detection. Anal Chim Acta 507:163–169
Goddard GR et al (2007) Analytical performance of an ultrasonic particle focusing flow cytometer. Anal Chem 79(22):8740–8746
Holmes D, Morgan H, Green NG (2006) High throughput particle analysis: combining dielectrophoretic particle focussing with confocal optical detection. Biosens Bioelectron 21(8):1621–1630. doi:10.1016/j.bios.2005.10.017
Knight JB et al (1998) Hydrodynamic focusing on a silicon chip: mixing nanoliters in microseconds. Phys Rev Lett 80(17):3863. doi:10.1103/PhysRevLett.80.3863
Kohlheyer D et al (2008) A microfluidic device for array patterning by perpendicular electrokinetic focusing. Microfluid Nanofluid 4(6):557–564. doi:10.1007/s10404-007-0217-9
Laulicht B et al (2008) Evaluation of continuous flow nanosphere formation by controlled microfluidic transport. Langmuir 24(17):9717–9726
Mao X, Waldeisen JR, Huang TJ (2007) “Microfluidic Drifting”-implementing three-dimensional hydrodynamic focusing with a single-layer planar microfluidic device. Lab Chip 7:1260–1262
McClain MA et al (2001) Flow cytometry of Escherichia coli on microfluidic devices. Anal Chem 73(21):5334–5338
Nieuwenhuis JH et al (2003) Integrated flow-cells for novel adjustable sheath flows. Lab Chip 3:56–61
Park SJ et al (2004) Rapid three dimensional passive rotation micromixer using the breakup process. J Micromech Microeng 14:6–14
Pollack L et al (2001) Time resolved collapse of a folding protein observed with small angle X-ray scattering. Phys Rev Lett 86(21):4962. doi:10.1103/PhysRevLett.86.4962
Shin S-J et al (2007) “On the fly” continuous generation of alginate fibers using a microfluidic device. Langmuir 23(17):9104–9108
Simonnet C, Groisman A (2005) Two-dimensional hydrodynamic focusing in a simple microfluidic device. Appl Phys Lett 87(11):114104
Sundararajan N et al (2004) Three-dimensional hydrodynamic focusing in polydimethylsiloxane microchannels. J Microelectromech Syst 13(4):559–567. doi:10.1109/JMEMS.2004.832196
Tung Y-C et al (2004) PDMS-based opto-fluidic micro flow cytometer with two-color multi-angle fluorescence detection capability using PIN photodiodes. Sens Actuators B 98:356–367
Umesh APS, Chaudhuri BB (2000) Some efficient methods to correct confocal images for easy interpretation. Micron 32(4):363–370
Wang Z et al (2004) Measurements of scattered light on a microchip flow cytometer with integrated polymer based optical elements. Lab Chip 4:372–377
Wolfe DB et al (2004) Dynamic control of liquid-core/liquid-cladding optical waveguides. Proc Natl Acad Sci 101(34):12434–12438
Zhao Y et al (2007) Optical gradient flow focusing. Opt Express 15(10):6167–6176
Zimmermann T (2005) Spectral imaging and linear unmixing in light microscopy. Adv Biochem Eng Biotechnol 95:245–265
Acknowledgments
This work was supported in part by the National Institute of Justice (NIJ #2004-DN-BX-K001) and the Ludwig Institute for Cancer Research. S.L.P. thanks the Intel Foundation and the National Nanotechnology Infrastructure Network Research Experience for Undergraduates (NNIN REU) Program. This work was performed in part at the Cornell NanoScale Facility, a member of the National Nanotechnology Infrastructure Network, which is supported by the National Science Foundation. Images were acquired at the Microscopy and Imaging Facility of the Cornell University Life Sciences Core Laboratories Center. M.J.K. thanks Anthony Reeves for discussions about the image processing and Carol Bayols for advice on the two-color imaging experiment.
Author information
Authors and Affiliations
Corresponding author
Appendix: Image processing
Appendix: Image processing
The experimental images were modified using two image processing procedures: spectral unmixing and depth-dependence compensation. An implementation of these procedures was written in MATLAB, and a detailed description is provided here for the interested reader.
The net effect of image processing is shown in Fig. 7. The spectral bleedthrough of rhodamine into the green-channel is present in the original image shown in Fig. 7a, but after image processing, the rhodamine appears purely red because the bleedthrough effect has been subtracted out of the processed image shown in Fig. 7b. Also, the original image is brighter near the top of the microchannel than near the bottom, but the brightness of the processed image is more uniform with depth because the image has been compensated for the depth-dependent response of the optical experiment.
Fluorescence micrographs and intensity profiles showing the effect of image processing. a Original image alongside a profile of the pixel green-values (solid line) and pixel red-values (dashed line) obtained along the vertical center of the image. The image pixels contained integer values between 0 and 255. In each of the pixel profiles, y varies along the vertical axis from y = 0 to y = 1. b Processed image; c original image, pixel green-values; d processed image, pixel green-values; e original image, pixel red-values; f processed image, pixel red-values
1.1 Spectral unmixing
A flat-field image was acquired in which all inputs to the flow-cell contained rhodamine. Ideally, the rhodamine would be detected as purely red and the pixel green-values of this image would be zero everywhere. However, rhodamine was detected not only by the red-channel photodetector but also by the green-channel photodetector, and therefore the pixel green-values were non-zero and were observed to be directly proportional to the red-values. Moreover, the fraction of the red bleeding into the green depended on depth. The factor of spectral bleedthrough along the depth of the channel, F S(y), was obtained by averaging the pixels horizontally and then dividing the average green-value of each row of pixels by the average red-value of each row of pixels. It was found that F S(y) was equal to 17% near the top of the channel and 10% near the bottom of the channel.
The effect of spectral bleedthrough was removed from each experimental image by subtracting the appropriate quantity from the pixel green-values according to the following formula, where I G and I R represent the pixel green-values and red-values of the original image and \( \tilde{I}_{\text{G}} \) represents the pixel green-values of the unmixed image:
Prior to the subtraction procedure, the pixel green-values near the top of the microchannel were brighter than background, as shown in Fig. 7c, but after the subtraction procedure, the pixel green-values were bright only in the region of the fluorescein-containing fluids, as shown in Fig. 7d. This straightforward subtraction technique provides a computationally simple implementation of linear unmixing and is possible in our case because only rhodamine was detected in both the red and the green whereas fluorescein was detected only by the green-channel photodetector (Zimmermann 2005).
1.2 Depth-dependence compensation
Depth-dependence compensation was required because the original images were bright near the top of the microchannel and dim near the bottom of the microchannel. This depth-dependence of image brightness did not reflect the concentration distributions of the fluids but resulted from uneven illumination along the depth of the specimen and imperfections of the optical detection system of the microscope.
The spatial decay of light intensity with depth has been encountered previously in three-dimensional confocal microscopy, and two competing methods have been used to correct this decay: image arithmetic and mathematical correction. The image arithmetic approach normalizes the images according to a formula originally developed for fluorescence microscopy (Park et al. 2004):
where OI represents the original image, CI represents the corrected image, DF represents the dark-field image in which no dye is present, and FF represents the flat-field image in which dye is uniformly present. This technique relies heavily on the precise alignment of the dark-field image to the flat-field and experimental images. In practice, however, it can be difficult to register the dark-field image to the experimental images because the walls of the microchannel cannot be detected in the absence of fluorescently stained fluids. For this reason, we elected to restore the images using a mathematical correction method.
Several alternative mathematical correction methods have been developed to correct confocal microscopy images suffering from the effect of depth-decay of brightness (Umesh and Chaudhuri 2000). Seeking ease of computation, we have developed a simple method of correction based on a linear fit and linear transformation. Our procedure begins by describing the decay of image brightness with depth based on the flat-field images. Two flat-field images were obtained: first, all of the fluid inputs to the flow-cell were loaded with rhodamine and a red flat-field image was acquired, \( I_{\text{R}}^{\text{FF}} \); second, all of the fluid inputs were loaded with fluorescein and a green flat-field image was acquired, \( I_{\text{G}}^{\text{FF}} \). A horizontal median filter was applied to the flat-field image in which fluorescein-labeled fluid was pumped into all of the inputs of the flow-cell by using the nlfilter() function of MATLAB with a sliding block of 20 × 1 pixels. Then, a profile was obtained vertically up the center, revealing a profile of the depth-dependence of the intensity of fluorescence, \( I_{\text{G}}^{\text{FF}} (y) \). The parameters describing the depth-dependence were obtained by fitting this profile with the following equation:
where a characteristic height was identified, y C, above which the image brightness was approximately constant and below which the brightness decayed approximately linearly, and where the fitting parameters m G and b G were obtained by linear regression. For the green flat-field image, \( I_{\text{G}}^{\text{FF}} (x,y) \), this decay occurred for \( y < y_{\text{G}}^{\text{C}} \), where \( y_{\text{G}}^{\text{C}} = 0.63 \) and y is measured from the bottom of the microchannel and is normalized between zero and one. The slope of decay was m R = −1.9 ± 0.1 μm−1, and the intercept at y G was b G = 217 ± 3; here, it is useful to recall that the pixel-values of the original image were 8-bit integers ranging from 0 to 255. The fitting procedure was repeated similarly for the red flat-field image, \( I_{\text{R}}^{\text{FF}} (x,y) \), and it was found in this case that \( y_{\text{R}}^{\text{C}} = 0.49 \), m R = −1.9 ± 0.1 μm−1, and b R = 213 ± 9. Restated, the brightness of the green flat-field image decayed from \( I_{\text{G}}^{\text{FF}} \approx 217 \)at a critical depth of 48 μm to \( I_{\text{G}}^{\text{FF}} \approx 61 \) near the bottom of the microchannel, with a constant rate of decay of 1.9 μm−1 at depths beyond than the critical depth. Similarly, the intensity of the red flat-field image decayed from \( I_{\text{R}}^{\text{FF}} \approx 213 \) at a critical depth of 66 μm to \( I_{\text{R}}^{\text{FF}} \approx 91 \) near the bottom of the microchannel, with a constant rate of decay of 1.9 μm−1. In the top portion of the image, above the critical depth, the brightness in each of the flat-field images was approximately constant.
With these parameters at hand, each experimental image was restored of depth decay by applying the following transformation independently to the red color-component of the image, I R(x, y), and the green color-component of the image, I G(x, y), where I′(x, y) is the corrected image:
Prior to the procedure, the pixel red-values were less bright near the bottom of the microchannel than near the top of the microchannel, as shown in Fig. 7e, but after the procedure, the pixel red-values near the top and bottom of the microchannel were of similar brightness, as shown in Fig. 7f.
Rights and permissions
About this article
Cite this article
Kennedy, M.J., Stelick, S.J., Perkins, S.L. et al. Hydrodynamic focusing with a microlithographic manifold: controlling the vertical position of a focused sample. Microfluid Nanofluid 7, 569 (2009). https://doi.org/10.1007/s10404-009-0417-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-009-0417-6